
* Along with the understanding of atoms, 19th century chemists managed to decipher the structure of simple molecules, and developed the ability to synthesize many molecules, sometimes on an industrial scale. Chemists would also take the first steps towards understanding the chemistry of life itself.

* With the rise of modern analytic chemistry, chemists were beginning to acquire a crude understanding of the chemistry of life. There had long been a belief that there was some magical "spark" of life. In the early days of electrical experimentation this was often identified as electricity, partly because Volta had conducted experiments in which he made the dismembered legs of a frog twitch by applying an electric shock, as if they had come alive again for an instant. It was nonsense in hindsight -- the electric spark simply stimulated nerves in the disconnected legs -- but this notion remains visible today in the many movies based on Mary Shelley's 1818 novel FRANKENSTEIN, in which Dr. Frankenstein uses the power of lightning to animate a monster put together from pieces of corpses.
More formally, this concept was enshrined in the notion of "vitalism", which had been promoted by Stahl in the previous century and was championed by Berzelius. Vitalism that the chemical products of biological systems -- which Berzelius called "organic" chemicals -- could only be produced by biological systems, though he did realize that organic chemicals could be "denatured" into inorganic chemicals. In effect, vitalism claimed that there was a dividing line between the chemistry of living and nonliving systems, which wasn't a completely unreasonable an assumption at the time since the chemistry of biological systems would prove far more complicated than could be imagined in those days.
However, in 1828, Friedrich Woehler (1800:1882), a student of Berzelius, heated what was believed to be an inorganic compound named ammonium cyanate, and found that it produced urea, a major component of urine. This was so startling that Woehler checked his results several times before releasing them, and even then they weren't believed by all. Berzelius only accepted Woehler's research when absolutely forced to by the data.
Woehler's discovery was unspectacular in itself, something that someone would have stumbled across sooner instead of later, but in 1845 a student of Woehler's, Adolph Wilhelm Hermann Kolbe (1818:1884), produced acetic acid, a primary component of vinegar, tracing its construction from individual atoms into the final molecule. This "primary synthesis" was a technical masterpiece, and it was followed by more of the same, with a French chemist named Pierre Eugene Marcelin Berthelot (1827:1907) performing primary synthesis on a large number of organic molecules, including methane, acetylene, benzene, methanol or wood alcohol, and ethanol or grain alcohol. The first steps had been taken that would eventually show that life processes were chemical processes, no more and no less.
The biological molecules synthesized to that time were still simple compounds, and dealing with more complicated biological substances remained troublesome. In 1812, a Russian chemist named Gottlieb Sigismund Kirchoff (1764:1833) had boiled starch obtained from plants in a dilute acid, and obtained a simple sugar molecule, which would become known as "glucose". In 1819, a French chemist named Henri Braconnot boiled plant fibers and also found they broke down into glucose. Obviously starches and plant fibers were made up of glucose subunits, possibly in the form of chains, but that was as far as the notion could be taken at the time.
In 1820, Braconnot performed much the same experiment on gelatin and obtained a simple compound that would be named "glycine". It was a nitrogen-containing organic acid, and further work on a would find over 20 similar compounds, which Berzelius would name "amino acids".
Exactly what to make of this discovery wasn't clear, but it had been known for decades that there was a class of biological materials, such as egg white ("albumen"), that had the contrary property of solidifying when heated. In 1839, a Dutch chemist named Gerardus Johannes Mulder (1802:1880) decided that these "albuminous" materials had a common basic chemical formula (though he was off-base in that regard) and, on a suggestion from Berzelius, named them "proteins", from Greek for "prime importance". Proteins turned out to be common biological materials -- gelatin was a protein -- and it was apparent that they were composites of amino acids. Proteins would prove to have diverse functions, operating as structural components, blood components, and -- in the form of "enzymes" -- as catalytic components.
Another French chemist, Michel Eugene Chevreul (1786:1889), performed studies of fats. He first started with soap, which was manufactured by heating fats with alkali. Chevreul treated soap with acid and came up with what would be called "fatty acids", and then showed that the conversion of fats to soap involved the removal of a compound known as "glycerol" from the fats. Analysis showed that glycerol was a simple molecule, which could serve as a "junction" for three other molecules. Apparently fats were simpler than starches or proteins. In 1854, Berthelot heated glycerol with "stearic acid", a common fatty acid, and produced a known fat named "tristearin". It was the most complicated biological molecule to be synthesized to that time. Berthelot went further, heating glycerol with other acids that were not obtained from biological sources, producing fats that did not exist in nature.
It had become obvious that there was no strong dividing line between organic and inorganic chemicals, making the notion of vitalism increasingly difficult to maintain. In fact, in 1861, the German chemist Friedrich August Kekule von Stradonitz (1829:1886), blessedly known simply as Kekule, redefined the term "organic" to mean almost all compounds based on carbon, excluding a few compounds like CO2, with the term "inorganic" applied to all the rest.
BACK_TO_TOP* While chemists were probing the molecules of life, they were also acquiring improved abilities to determine the structure of molecules. In 1831, Baron Justus von Liebig (1803:1873) conducted careful experiments in which he burned organic substances and collected the gases released, which turned out to be chiefly water vapor and carbon dioxide. Careful measurement of the weights "before and after" revealed the composition of a number of substances, prominently including ethyl alcohol or ethanol, which von Liebig determined had the composition C2H6O.
Von Liebig's tricks couldn't measure the proportion of nitrogen in organic substances, but in 1833 the French chemist Jean Baptiste Andre Dumas (1800:1884) came up with a scheme for collecting the nitrogen released by combustion of such materials. Using the improved tools, in 1841 he also performed an analysis of the composition of the atmosphere with unprecedented accuracy.
However, as the general composition of more and more compounds was determined, chemists began to become baffled, since in many cases they found two different compounds with entirely different characteristics that had the same general composition. In the 1820s, Woehler was performing studies of the composition of a class of chemicals known as "isocyanates", while von Liebig was performing studies of another class of chemicals known as "fulminates". They submitted papers to a chemical journal more or less simultaneously, and the editor realized the two researchers had derived exactly the same general composition for two generally different chemicals.
Von Liebig and Woehler ended up at odds over the matter, but they talked things out and became close collaborators. As it turned out, they were both right. Although it could be believed up to that time that compounds were simple agglomerations of atoms, like the ingredients of a cake that could be mixed together, it was becoming clear that the atoms had to be plugged together in a specific structural arrangement. Molecules with the same general composition but different structures were named "isomers".
* There remained the question of how to figure out these structures. For example, methane had the general formula of CH4. That suggested a range of possible structures, for example:
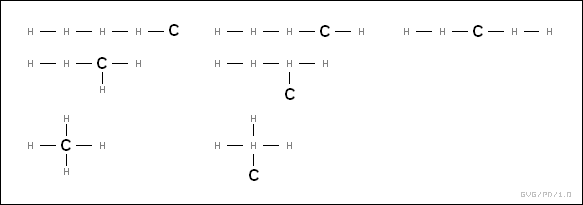
How could chemists sort between the different possibilities?
One aid turned out to be that when all but the simplest molecules were broken up, they usually didn't simply come apart into constituent atoms, but produced distinctive fragments. Barium carbonate or BaCO3, for example, broke into Ba and CO3, while strontium sulfate or SrSO4 broke into Sr and SO4. The distinctive fragments such as carbonate, CO3, and sulfate, SO4, were called "radicals", or later "groups".
Berzelius liked the notion of radicals, but acquired some ideas that are hard to understand in hindsight: he thought that radicals could only contain carbon and hydrogen, never elements like oxygen -- not true -- and that the radicals had certain specific positive or negative voltages associated with them -- which was sort of true, but Berzelius came up with tables of radicals and their corresponding voltages that would prove unworkable.
In 1836, a student of Dumas named Auguste Laurent (1807:1853) substituted chlorine for some of the hydrogen atoms in ethanol, which violated Berzelius' schemes. Berzelius attacked Laurent's results and did what he could to block Laurent's personal advancement, but after the death of Berzelius in 1848, Laurent's notions became accepted wisdom. Laurent abandoned the tables of voltages, in effect saying that the basis of chemical bonding was a problem for a later generation, and instead focused on a system of organizing organic molecules. Laurent considered the water molecule, H2O, and wondered what would happen if a hydrogen were removed, resulting in what is now called the "hydroxyl" radical, OH, and replaced by a "methyl" radical, CH3, or an "ethyl" radical, C2H5. The results were methanol, CH3OH, and ethanol, C2H5OH. In Laurent's system, all such recombinations with the hydroxyl radical were alcohols, and in fact all alcohols had similar properties (though only ethanol was actually safe to drink).
In the meantime, an English chemist named Alexander William Williamson (1824:1904) was considering what would happen if both hydrogens in the H2O molecule were replaced by organic radicals. The result were the "ethers", such as C2H5OC2H5, which is the "ether" once commonly used as a general anesthetic. Similarly, a French chemist named Charles Adolf Wurtz (1817:1884) investigated what would happen if the hydrogens of the ammonia molecule, NH3, were replaced by organic radicals; the results were called "amines". By 1880, compilations of organic compounds listed huge numbers of entries, organized by Laurent's system.
* Figuring out the precise structures of complicated molecules was still difficult, but chemists were beginning to realize that different elements would combine with others only in very specific ways. For example, oxygen would combine with two hydrogen atoms to form water, H2O; nitrogen would combine with three hydrogen atoms to form ammonia, NH3; carbon would combine with four hydrogen atoms to form methane, CH4. It was as though elements were analogous to wooden balls with sockets in them that could accommodate pegs to link or "bond" them to other balls. Each type of element had a specific number of sockets: hydrogen, it seemed, had one socket, oxygen two, nitrogen three, and carbon four. In some cases, "double bonds" -- two connections between two atoms -- or "triple bonds" -- three connections -- could be formed.
The first person to clearly articulate this notion was an English chemist named Edward Frankland (1825:1899), who published his ideas in 1852. He called the bonds "valence" bonds, after the Latin word for "power". Kekule picked up Frankland's idea and ran with it, using a suggestion by a Scots chemist named Archibald Scott Couper (1831:1892) to create simple "structural diagrams". That meant that the actual structure of methane was a central carbon atom flanked by four hydrogen atoms. The knowledge also made clear the structures of other simple molecules, such as ammonia (NH3) and water (H2O):
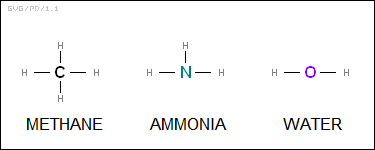
These simple molecules all feature single bonds, but in some cases, molecules could be joined by "double bonds" or even "triple bonds". Kekule published a landmark textbook in 1861 that helped establish the scheme.
Structural diagrams helped unravel the mystery of isomers. In the 1860s, a Russian chemist named Alexander Mikhailovich Butlerov (1828:1886) showed that while ethanol and dimethyl ether had the same composition -- C2OH6 -- they had different structures, as follows:
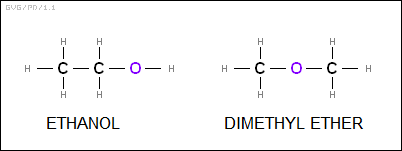
One of the puzzles of chemical structures at the time was the nature of the carbon-based chemical benzene, C6H6. Nobody could figure out any way to fit this assembly together in a way that followed "the rules" as they were known at the time. In a famous little story, Kekule figured out the structure of benzene in 1865 while he was dozing on a London omnibus, visualizing snakes in a ring, eating each other's tails. Benzene consisted of six carbon atoms in a hexagonal ring, with alternating single and double bonds and hydrogen atoms mated to each of the carbon atoms on the outside of the ring. The scheme worked -- though by the knowledge of the time the extraordinary stability of the benzene ring seemed mysterious, since double bonds had a tendency to break apart and form new single bonds in reactions.
BACK_TO_TOP* Structural diagrams were a major advance, but they were only part of the story of molecular structures. A further understanding of molecular structures would be driven by a seemingly obscure phenomenon known as "optical isomerism".
It had been known since early in the 19th century that light was a wave phenomenon, and could be "polarized" so that its wave oscillations took place in a particular plane along the motion of a light beam -- the light beam could be vertically polarized, horizontally polarized, or polarized at any angle in between. In 1815, a French physicist named Jean Baptiste Biot (1774:1862) showed that certain crystals, significantly including those of some organic compounds, could rotate the polarization angle of a beam of light. Sometimes the rotation was clockwise or right -- "dextrorotation" -- and sometimes it was counterclockwise or left -- "levorotation".
In some cases, the optically active organic crystals seemed to be identical except for the direction in which they rotated the polarization of a light beam. In 1848, Louis Pasteur (1822:1895) -- another of the great French chemists, the intellectual offspring of Lavoisier -- conducted a careful analysis of the crystals of an optically-active material, sodium ammonium tartrate. He noticed that the crystals of the material seemed to have "handed" symmetry, with some being left-handed and some right-handed. Since the optical activity persisted even when the crystals were dissolved in solution, Pasteur concluded that the asymmetry must persist down to the molecular level. In other words, there were isomers that had distinctive "left-hand" and "right-hand" structures, a concept now known as "chirality".
Pasteur's work turned out to have relevance to the three-dimensional structure of molecules. Structural diagrams were two-dimensional; few could seriously believe that molecules laid themselves out neatly in a plane, but figuring out the three-dimensional structures was a challenge. In 1874, a Dutch graduate student named Jacobus Hendricus Van't Hoff (1852:1911) was working towards his doctorate when he proposed that the four bonds of the carbon atom were distributed symmetrically in three dimensions, as if the carbon atom were in the center of a tetrahedron -- the simplest solid made up of planes, composed of four triangles mated together -- and the bonds were oriented towards the vertices of the tetrahedron, with each bond at an angle of 109.5 degrees to the others. This arrangement ensured the maximum separation of the four groups around the carbon atom.
In this view, the methane molecule, CH4, consisted of a carbon atom at the center of the tetrahedron and the four hydrogen molecules at the corners. What made this model particularly appealing was that it explained the optical isomerism of certain organic molecules, such as lactic acid, which also had a tetrahedral structure, but featured different groups at each vertex of the tetrahedron. The possible arrangements of the four groups resulted in two structures that had all the same parts but were mirror images of each other, resulting in distinctive left-hand and right-hand three-dimensional molecular structures, or "stereoisomers", that produced the desired optical behavior.
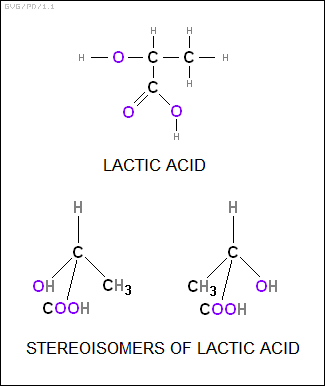
A French chemist named Joseph Achille Le Bel (1847:1930) came up with the same idea in parallel, and so the concept of the tetrahedral carbon-based molecule is sometimes called the "Van't Hoff / Le Bel theory". The idea was accepted almost immediately, since it was not only elegant, it also explained the structure and behavior of a wide range of organic molecules.
* It was the leading edge of a wave of discoveries. A German chemist named Viktor Meyer (1848:1897) came up with a comparable description of the three-dimensional structure of simple nitrogen-based molecules. Nitrogen has a valence of three; in Meyer's view, the ammonia molecule, NH3, consisted of a tetrahedron with the nitrogen at the top and the hydrogen atoms at the other three vertices of the tetrahedron. Meyer's work also showed how some nitrogen-based molecules acted as optical isomers.
An English chemist named William Jackson Pope (1870:1939) came up with explanations for the three-dimensional structures of simple molecules based on sulfur, selenium, tin, and other atoms; and a Swiss-German chemist named Alfred Werner (1866:1919) established structures for simple molecules based on cobalt, chromium, rhodium, and a number of other metals. Werner extended the idea by arguing that, for some relatively complicated inorganic molecules, certain geometric patterns could account for the distribution of the groups of the atoms around a central atom. Werner's "coordination chemistry" would ultimately be proven correct, winning him the Nobel Prize for chemistry in 1913.
These ideas were extended to even explain the benzene ring. In 1885, a German chemist named Johann Friedrich Wilhelm Adolf von Baeyer (1835:1917) pointed out that since the orientations of bonds in a tetrahedral carbon-based molecule like methane were at an angle of 106.5 degrees to each other, then ring-based carbon structures where the bond angles were comparable would be more stable than those in which the bond angles were much narrower, causing more "strain".
Three carbon atoms linked into a triangle wasn't very likely, since the bond angle would then be only 60 degrees, and a square arrangement of four carbon atoms was only somewhat better, with bond angles of 90 degrees. However, a five-carbon ring had bond angles of 108 degrees, which was very tolerable, and a six-carbon ring, the benzene ring, had bond angles of 120 degrees, which was almost as tolerable. It was yet another one of the chemists' many rules of thumb, but it accounted for the fact that five-carbon and six-carbon rings were common in organic chemistry, while other ring configurations were rare.
* While some chemists were beginning to understand the nature of molecules, others were developing tools for analysis of those molecules. The Scots chemist Thomas Graham (1805:1869) performed studies in "diffusion", the gradual spread of atoms or molecules released into a liquid or gas. He initially focused on the diffusion of gases through small holes or narrow tubes, and published a result in 1831 that the rate of diffusion of a gas was proportional to the square root of its molecular weight. This observation became known as "Graham's law".
Graham then went on to investigate diffusion in solutions divided by a paper membrane. Some solutes, like salt, sugar, and copper sulfate would diffuse through the porous paper separator; Graham called such solutes "crystalloids". Others, like glue or gelatin, wouldn't diffuse through the separator; he called them "colloids". He was effectively the founder of what is now called "colloid chemistry".
In the course of his studies, Graham discovered the process of "osmosis". If a selectively semipermeable membrane was used to separate two volumes of water, and a solute that wouldn't pass through the membrane was dissolved in one volume, the concentration of water (as a proportion of the solution) would be less on that side of the membrane. That meant that water was more likely to pass into that side through the membrane than back out across the membrane again, with the result that the total volume of the solute on one side of the membrane would increase at the expense of the volume of the pure water on the other side of the membrane, incidentally creating an "osmotic pressure" across the membrane. In 1877, a German botanist named Wilhelm Pfeffer (1845:1920) showed that osmotic pressure could be used to determine the molecular weights of the solute. It was the first workable technique for the measurement of the masses of large molecules.
BACK_TO_TOP* Although large-scale chemical industries had existed for centuries, one of the most significant being the manufacture of black powder, the 19th century saw an industrial revolution in industries based on chemical technologies. New, much more powerful explosives were developed, as were methods of producing steel on a large scale; photographic techniques based on light-sensitive silver-based dyes were invented as well.
Chemists were also able to come up with synthetic chemicals that were the basis for what would become massive chemical industries. In 1856, an English chemistry student named William Henry Perkin (1838:1907) was attempting to synthesize the anti-malarial drug quinine, on the suggestion of his professor, August Wilhelm von Hofmann (1818:1892), a German chemist teaching in England. The starting material was "coal tar", a nasty black gummy material bled off by coal heated in the absence of air to produce "coal gas" for heating; part of the interest in chemical processing of the material was simply to find a convenient way to get rid of it. Hofmann had used coal tar as a feedstock for synthesizing benzene and "aniline", a similar substance that contained nitrogen.
Perkin had no chance of synthesizing quinine, since it had a relatively complicated structure that was not understood, and it wouldn't be synthesized until the next century. However, the young Perkin, still a teenager at the time, tinkered with one of his dead ends and found out that if he couldn't synthesize quinine, he could at least come up with a spectacular purple dye based on aniline. He went into business to manufacture and sell his "aniline purple", quickly becoming rich. Up to that time, dyes had been obtained from plants and other organisms, which made scaling up production difficult. Synthetic dyes could be manufactured cheaply on an industrial scale.
The word got out immediately that there was far more gold in the creation of synthetic dyes than any alchemist ever got out of lead, and many chemists jumped into the field. Hofmann himself synthesized a set of popular violet dyes, going back to Germany in 1865 to help establish the German chemical industry. Perkin became so wealthy that he managed to quit business at age 35, returning to chemical research, leading the way on the synthesis of chemicals for perfumes.
By the end of the century, chemical synthesis was big business, with factories churning out new dyes, drugs, explosives, and what would evolve into the modern plastics industry. Germany in particular embraced the "chemical industrial revolution", becoming the world's powerhouse for the manufacture of such materials. Other chemists continued to unravel the composition of biological chemicals, also establishing the basis for a revolution in biochemistry.
* One of the more ironic results of the revolution in practical chemistry was the revival of alchemy in its most fraudulent form. With chemists able to perform such seeming miracles of chemical synthesis, it wasn't so implausible, at least to the scientifically illiterate, that chemists might be able to actually achieve the dream of transforming lead into gold. A number of confidence artists exploited this gullibility by creating a "hyperchemistry" that could achieve such miracles, and even as late as the 1890s some of them managed to make off with a considerable haul of cash before the "marks" realized they'd been had.
BACK_TO_TOP* Organic chemistry, the study of carbon-based compounds, is an elaborate field in itself, and it's not possible to do more than give a sketchy survey of it here. Organic chemistry covers most fuels, plastics and synthetic fibers, as well as drugs and various classes of biomolecules -- though biologically active organic molecules are more generally discussed as part of biochemistry.
Organic molecules are organized into a number of classes. The best-known are hydrocarbons, which are composed of nothing but carbon and hydrogen -- they are not to be confused with "carbohydrates", by the way, which are discussed later. There are several subclasses of hydrocarbons, the simplest being the "alkanes", which are straight or branch-chained molecules, all joined with single bonds. The simplest alkane is methane (CH4), followed by ethane (C2H6), propane (C3H8), butane (C4H10), pentane (C5H12), hexane (C6H14), heptane (C7H16), octane (C8H18), and so on.
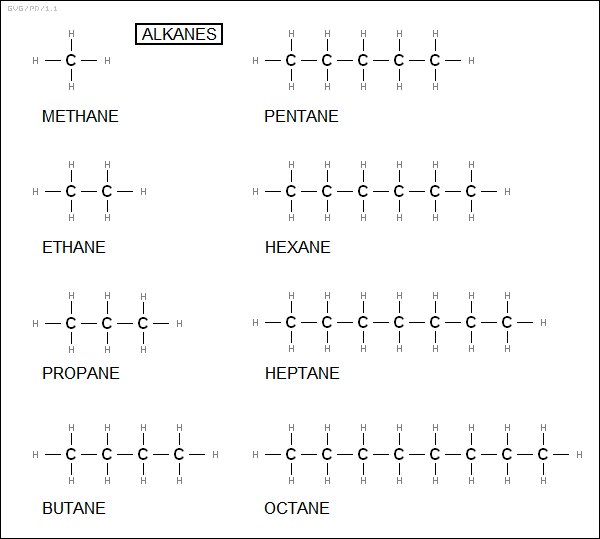
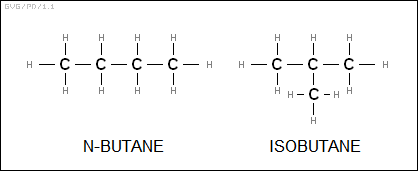
The general formula for an alkane is obviously "C<N>H<2*N+2>", which would seem simple enough, but there's a complication: butane can have two isomeric configurations, either with a chain of four carbons, known as "normal butane" or "n-butane", or a chain of three carbons with one carbon off to the side, which is "isobutane". The larger an alkane gets, the more isomers it can have; octane (C8H18), for example, has 18 isomers, and in principle C20H42 has over 350,000 isomers. There is an IUPAC scheme for naming the different isomers, featuring a numbering scheme to designate the location of side chains -- for example "2,2,4-trimethylpentane" -- but the details are only really of interest to chemistry professionals. All the following families also have IUPAC nomenclature systems, though they won't be discussed in any detail.
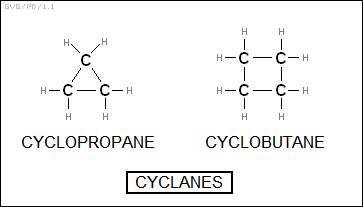
The "cycloalkanes" or "cyclanes" are effectively alkanes looped into rings. The smallest ring consists of three carbons, making it "cyclopropane", with four carbons giving "cyclobutane", and so on. The general formula for cyclanes is "C<N>H<2*N>".
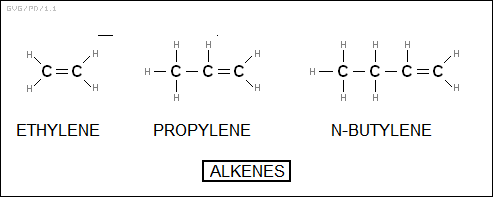
* The general formula "C<N>H<2*N>" also applies to another subfamily of hydrocarbons, the "alkenes", which are like the alkanes but have one double bond between carbon atoms. The naming has some similarities to that of the alkanes, staring with "ethylene" (C2H4), then "propylene" (C3H6), "butylene" (C4H8), and so on. There is a different IUPAC naming scheme in which these molecules are "ethene", "propene", and "butene" respectively. Of course, there are many isomers of alkenes, with the isomeric configurations defined not only by the position of branched chains but of the double bond, and an associated complicated IUPAC naming scheme, for example "3,4-dimethyl-2-hexene".
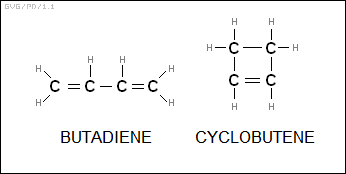
A hydrocarbon with two double bonds is an "alkadiene", with the general formula of "C<N>H<2*N-1>", for example "butadiene" (C4H9). The alkenes can also be joined back to themselves in loops, forming "cycloalkenes", for example "cyclobutene (C4H6)". These have the general formula of "C<N>H<2*N-2>".
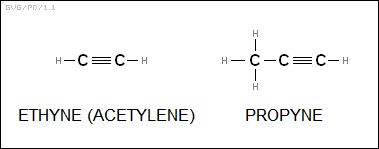
* Yet another major family of organic molecules are the "alkynes" or "acetylenes", which feature triple bonds between carbons and, like the cycloalkenes, have the general formula of "C<N>H<2*N-2>". The best-known example is "acetylene" (H2C2), used as a gaseous fuel in welding; it is also known as "ethyne". Other members of the family include "propyne" (C3H4), "butyne" (C4H6), and so on.
* These straight-chained and branched hydrocarbons are sometimes collectively referred to as "aliphatic" hydrocarbons. Aliphatic hydrocarbons with double or triple bonds tend to engage in reactions to increase their number of hydrogens at the expense of their multiple bonds, or in chemical terms these hydrocarbons tend to become more "saturated". In such terms, alkanes are "saturated" hydrocarbons while alkenes and alkynes are "unsaturated" hydrocarbons.
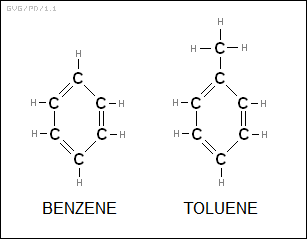
However, if six carbon atoms with three double bonds form into a ring, the result is a very stable molecule. The unadorned ring forms the "benzene (C6H6)" molecule, which is the basis for an entire family of ring-based hydrocarbon molecules, known as the "aromatic" hydrocarbons. Of course there are indefinite variations on a theme; for example "toluene (C7H8)" is a benzene ring with a CH3 (methyl) group attached.
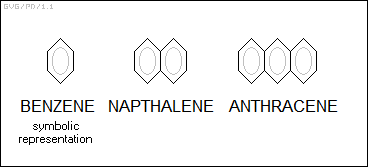
Benzene rings can also be linked together. "Napthalene (C10H8)" is two benzene rings linked together, while "anthracene (C14H10)" is three benzene rings linked together. They are found in coal tars and are used in the manufacture of synthetic dyes.
* While plugging together carbon and hydrogen atoms is good fun and, as the comments above indicate, can lead to an amazing variety of structures, of course there's no reason to refuse admittance to other atoms in organic chemistry. There are a number of classes of organic molecules defined simply by attaching a "functional group" of atoms based around oxygen, nitrogen, chlorine, or whatever.
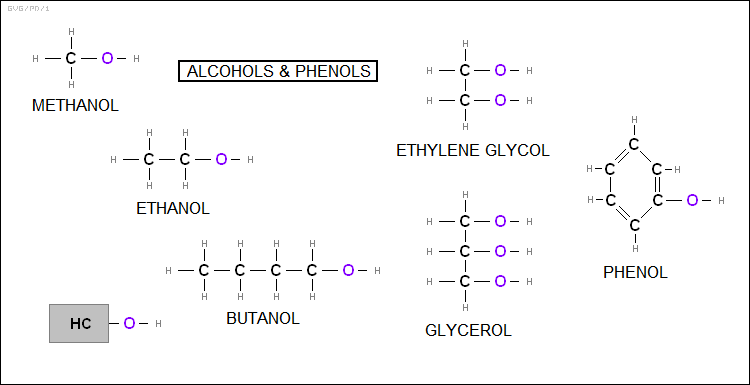
The "alcohols" are aliphatic hydrocarbons in which a hydroxyl (-OH) group is attached, replacing a hydrogen. If a hydroxyl group is attached to methane (CH4), the result is "methyl alcohol" AKA "methanol (CH3OH); if the hydroxyl group is attached to ethane (C2H6), the result is "ethyl alcohol" or "ethanol (C2H5OH)" AKA "grain alcohol", it being the alcohol produced by fermentation that gets us drunk. Both are used as biofuels. Other examples of alcohols include:
The "phenols" are similar, with a hydroxyl group attached to an aromatic hydrocarbon. If a hydroxyl group is attached to benzene (C6H6), the result is simply "phenol (C6H5OH)".
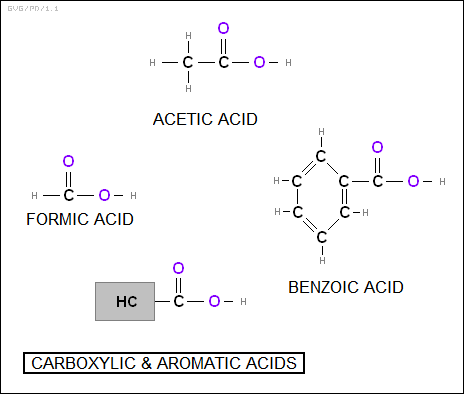
Attachment of a "carboxyl" group (-COOH) to an aliphatic hydrocarbon produces the "carboxylic acids". Simple examples are "formic acid (HCOOH)", found in biting ants, and "acetic acid (CH3COOH)", found in vinegar. Similarly, attaching a carboxyl group to an aromatic hydrocarbon yields an "aromatic acid", for example "benzoic acid (C6H5COOH)", the simplest member of the family.
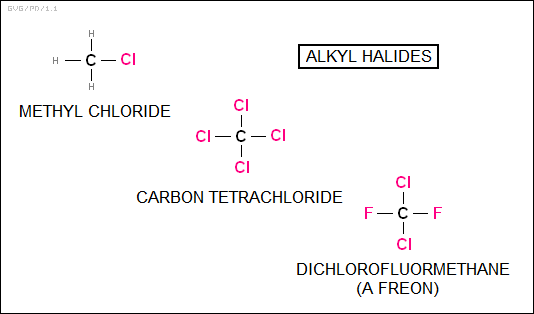
The "alkyl halides" consist of a hydrocarbon with a halogen -- fluorine, chlorine, bromine, or iodine -- attached. A simple example is "methyl chloride (CH2Cl)". Some are well known: "carbon tetrachloride (CCl4)" was once a common cleaning fluid, and "dichlorodifluoromethane (CCl2F2)" is one of the simpler members of the class of refrigerants called "freons".
Heating an alcohol and an organic acid together results in a combination called an "ester", essentially two hydrocarbons linked by a COO group. One common example is "ethyl acetate" or "CH3COOC2H5", which is used in nail polish remover, giving its distinctive smell. Other simple esters give various fruits their distinctive odors and are often used as artificial flavorings. For example, "amyl acetate" or CH3COOC5H11 gives the odor of bananas, while "ethyl butyrate" or C3H7COOC2H5 has the pineapple odor. Fats (and their liquid forms, oils) are esters of glycerol and organic acids; they are also referred to as "triglycerides".
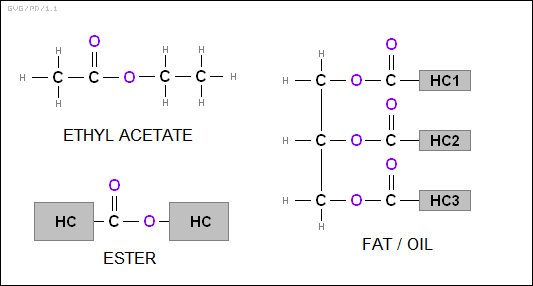
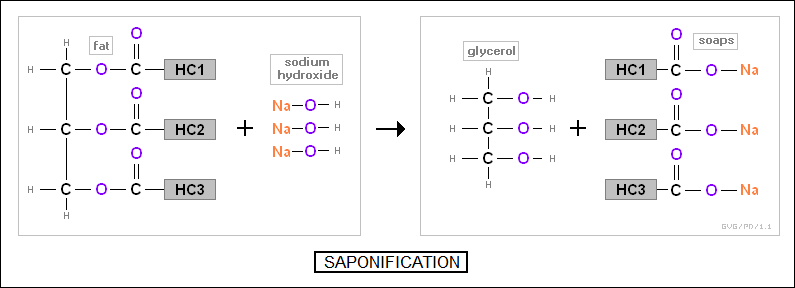
Incidentally, fats can be broken down by reacting them with a solution of sodium hydroxide (NaOH), resulting in glycerol and molecules of "soaps", which are hydrocarbons that end in a -COONa group. This process is known as "saponification". Soaps in solution form polarized anions that help dissolve grease, surrounding grease droplets with a "micelle" of anions -- with the nonpolar end of each molecule inward and the polarized end outward. This allows the grease particle to be suspended in the solution and then washed away.
It is also possible to form esters by combining alcohols and inorganic acids. One of the best-known cases is "nitroglycerine", which is formed from the reaction of glycerol and nitric acid:
C3H5(OH)3 + 3HNO3 -- H2SO4 --> C3H5(NO3)3 + 3H2O
This is not a chemistry experiment that should be performed at home by anyone who wants to have a home left.
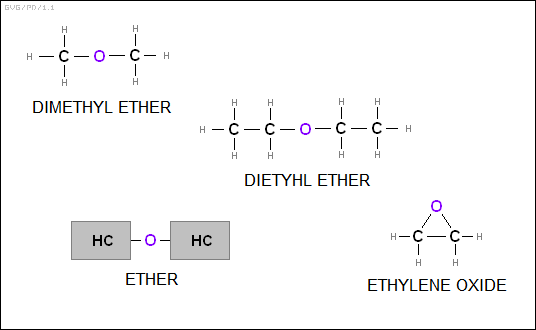
* The "ethers" consist of two hydrocarbons linked by an oxygen atom. A simple example is "dimethyl ether (CH3OCH3)", which has been used as an aerosol spray propellant. Most older people associate the word "ether" with a general anesthetic with a distinctive smell, with this molecule being "diethyl ether (C2H5OC2H5)", though it is no longer in serious use as an anesthetic. There are also "cyclic ethers", in the form of carbon-based rings with an oxygen atom in them, such as "ethylene oxide (C2H4O)".
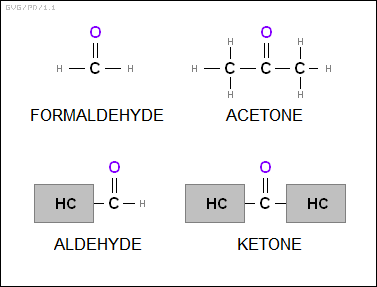
* The "aldehydes" consist of a "carbonyl" group (-COH) linked up to a hydrocarbon. The simplest example is of course "formaldehyde (HCHO)". The "ketones" are similar, but consist of two hydrocarbons linked by a carbonyl group. The simplest example is "acetone (CH3CCH4)".
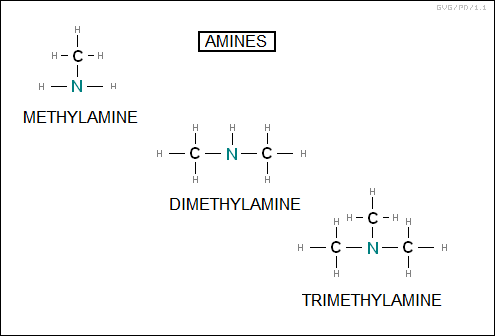
The "amines" are effectively an ammonia molecule (NH3) in which one or more of the hydrogens have been replaced by a hydrocarbon. There are "primary", "secondary", and "tertiary" amines, depending on whether one, two, or all three hydrogens have been replaced by hydrocarbons. The simplest examples of primary, secondary, and tertiary amines are "methylamine (CH3NH2)", "dimethylamine (CH3NHCH3)", and "trimethylamine (CH3N2(CH3))" respectively.
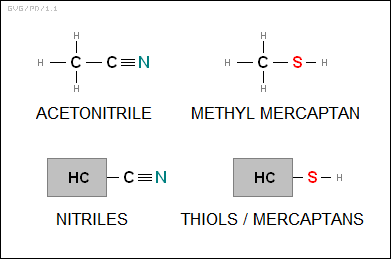
* The "nitriles" are hydrocarbons with a "nitrile" or "cyanide" group (-CN, the two atoms linked by a triple bond) attached. A simple example is "acetonitrile (CH3CN)". There are also "isontriles", in which the connection of the cyanide group to the hydrocarbon is reversed, with the link through the nitrogen atom and a triple bond to the carbon atom. The isonitriles are notorious for their absolutely foul dead-fish odor, though not all of them do smell bad.
The "thiols" or "mercaptans" are hydrocarbons with a "sulfhydryl" group (-SH) attached. A simple example is "methyl mercaptan (CH3SH)". Mercaptans are also notoriously foul-smelling, rank even in molecular quantities, and the basis for halitosis and skunk spray.
* As this discussion suggests, the possible variations of organic molecules are effectively endless. Categorizing them is a little like trying to categorize all the possible structures that could be assembled from a given set of Lego blocks. This is just a quick tour through the zoo; a detailed treatment can fill up a thick textbook of its own, but it would only be of interest to chemistry professionals.
The general feedstocks for organic chemicals are fossil fuels -- natural gas, petroleum, and coal. With fossil fuel supply beginning to tighten, there has been interest in biological feedstocks, such as sugar cane and algae.
BACK_TO_TOP