
* The discovery of the elements and the understanding of their properties led to one of the great scientific discoveries of the 19th century: Dmitriy Mendeleev's periodic table, which provided a tidy organizing system that would help point to a deeper understanding of the nature of matter. The later decades of the century also helped put chemistry on a sound theoretical basis through the development of "physical chemistry", in which chemical principles were linked to basic physics.

* The increasing number of elements and improved understanding of their properties led chemists to consider schemes for organizing the elements by those properties. In 1829, a German chemist named Johann Wolfgang Dobereiner (1780:1849) noticed that the three elements chlorine, bromine, and iodine seemed to have similar properties, with bromine being intermediate in weight between chlorine and iodine. Dobereiner noticed other "triads" along this line, such as calcium, strontium, and barium. Nobody paid him too much mind at the time, since gaps in the knowledge of elements, plus the poor knowledge of atomic weights and other properties of the elements, made his scheme of triads too haphazard to be very useful.
By the 1860s, chemists had much better knowledge of the elements and their properties, giving them a better basis for organizing the elements into a consistent scheme. In 1864, an English chemist named John Alexander Reina Newlands (1837:1898) constructed a table of the elements, but few chemists were impressed: although his table did manage to organize some elements by common properties, other elements were forced into the table arbitrarily, and the general reaction was that Newlands was simply finding coincidences in what amounted to "noise".
Newlands was actually on the right track, but the task of finally assembling a table that worked fell into the hands of a Russian chemist named Dmitry Ivanovich Mendeleev (1834:1907). In 1869, Mendeleev published a table that arranged the elements by weight in successive rows while ordering them according to common or "periodic" properties, particularly valence, by column. However, instead of trying to rigidly shoehorn the elements into the "periodic table" by weight, he had the self-confidence to arrange them by properties when the weights didn't seem to click properly -- assuming that it was the weights that were off, and that they would be adjusted by improved experiments.
Mendeleev was also confident enough to simply leave entries in the table blank when there didn't seem to be any good candidates, suggesting that the blank entries represented then-unknown elements. He then boldly proposed three undiscovered elements from the logic of the table, which he called "eka-boron", "eka-aluminum", and "eka-silicon", where "eka" was the Sanskrit word for "one", implying that the new elements were only one position in the table away from boron, aluminum, and silicon. The three elements were discovered over the following years:
No more ringing endorsement of Mendeleev's concepts could be conceived, and he remains one of the giants of chemistry. His table turned out to be no arbitrary scheme of pigeonholing the elements; it provided not only a system that mirrored what the next century would show to be the underlying internal structure of atoms, but also predicted the existence of previously unknown elements from vacancies in the table. Mendeleev's creation was a perfect example of a strong scientific theory, yielding both useful organization of information and predictive power. A German chemist named Julius Lothar Meyer (1830:1895) actually came up with a scheme similar to Mendeleev's in parallel, but Meyer failed to publish until 1870, resulting in a long priority dispute. Today, Mendeleev is generally credited as the inventor of the concept.
BACK_TO_TOP* Medeleyev's periodic table was quickly extended. In 1794, a Finnish chemist named John Gadolin (1760:1852) had discovered a metallic oxide, or "earth", that he named "yttria" after the quarry in which it had been found. It was only present in low concentration, and so he called it a "rare earth". It would take five decades to isolate an element from yttria, with the element of course named "yttrium".
There was nothing particularly startling about yttrium in itself. What was startling was that a series of elements were found that were obviously distinct from but extremely similar to yttrium. In the late 1830s and early 1840s, a Swedish chemist named Carl Gustav Mosander (1797:1858) discovered four such rare earths: "lanthanum", "erbium", "terbium", and "didymium". Actually, they turned out to be five rare earths. An Austrian chemist named Carl Auer, Baron von Welsbach (1858:1929) discovered in 1885 that there was really no such thing as didymium: it was a combination of two rare earths, "praesodymium" and "neodymium".
That wasn't the end of the list, either. Lecoq de Boisbaudran, discoverer of gallium, came up with two new rare earths, "samarium" (in 1879) and "dysprosium" (in 1886). A Swedish chemist named Per Theodor Cleve (1840:1905) discovered the rare earths "holmium" and "thulium" in 1879, and a French chemist named Georges Urbain (1872:1938) discovered the rare earth "lutetium" in 1907. That created a group of 14 elements of consecutive atomic weights but almost identical properties, so similar to each other it was difficult to separate them. There was now an entire set of elements that didn't fit conveniently into the periodic table, and the answer to the puzzle wouldn't be found in Mendeleev's lifetime.
* Mendeleev's table also turned out to be missing an entire column of elements, which was more or less discovered by a Scots researcher named Sir William Ramsay (1852:1916). Ramsay's discoveries were based on the new technique of "spectroscopy", which was established by two German physicists, Robert Wilhelm Bunsen (1811:1899), and Gustav Robert Kirchhoff (1824:1887).
Traditionally, materials that emitted light were seen as generating light of all colors -- as shown by the rainbow produced by passing light through a prism -- but in the 19th century physicists discovered that hot gases only emitted light of certain colors. If the light from a hot gas was passed through a prism, it resulted in a few "lines" of light instead of a rainbow. This was the "line emission spectrum" of the gas. If light from a hot solid object, which did generate a rainbow of light, was passed through a gas, the gas would similarly absorb light of specific colors, resulting in dark lines in the rainbow. This was the "line absorption spectrum" of the gas.
The significant feature of this phenomenon was that the line spectra were distinctive for gases of different elements, or in other words the line spectra could be used as a "fingerprint" of different elements. Spectroscopy would become an extremely important tool in chemistry, in particular helping to determine the chemical composition of the distant stars, a feat that had been previously judged impossible.
Kirchoff and Bunsen developed the first formal "spectroscope" to observe line spectra, and used it to discover the element "cesium" in 1860 and the element "rubidium" in 1861. They developed a procedure in which small samples of materials were vaporized in a hot burning gas jet for spectroscopic analysis. The hot gas jet was produced by a simple burner pipe developed by Bunsen, and the "Bunsen burner" would become his legacy to generations of chemistry students.
In 1882, the British physicist Lord Rayleigh (1842:1919), born Robert John Strutt, was analyzing atomic weights when he compared the density of nitrogen found in the air with nitrogen released from chemical reactions, and found that the gas obtained from air was denser. Like Cavendish, he suspected there was something else there, but unlike Cavendish, he had the tools to find out what it was. Ramsay helped Lord Rayleigh examine the residual gas using spectroscopy, and found that the gas had a spectrum unlike that of nitrogen. It was a new element, the inert gas "argon". In the 1890s, Ramsay extended this exercise to find four more inert gases: "helium", "neon", "xenon", and "krypton".
The spectrum of helium had actually been observed in the light of the Sun decades earlier, but claims that it was some new element did not go very far. The solar spectrum had lots of odd features, most of which turned out to be due to ions of perfectly familiar elements such as iron, and reading too much into it wasn't wise. The named "helium" had been assigned to the element in the spectral studies, the "-ium" ending being assigned on the basis that the element seemed to be a metal. It isn't, but the name stuck.
When Ramsay announced the discovery of argon and helium, they didn't fit into the periodic table as it was known, and chemists, including Mendeleev, were skeptical. Ramsay replied as his contribution to the long-standing friction between chemists and physicists with the comment that in physics "the second-rate men seem to know their place!" In the end, the existence of a column of nonreactive elements was easy to accommodate: the column was simply tacked on to the right side of the periodic table. These elements became known as the "noble gases", since they were so unreactive.
BACK_TO_TOP* As noted earlier, the tension between chemists and physicists was historically rooted in the perception that chemistry was merely a collection of "cookbook" rules whose deep physical basis was obscure. There were chemists who found this state of affairs unsatisfactory and wanted to establish chemistry on a firmer physical basis, by performing exacting measurements and developing a more rigorous theoretical structure for the understanding of chemical reactions. The result of these efforts was the creation of a new branch of chemistry known as "physical chemistry".
During the 19th century, a number of physicists had established the branch of physics known as "thermodynamics", the study of heat and its transformations. In the 1840s the pioneers in this effort -- including the British physicist James Prescott Joule (1818:1889) plus two German physicists, Julius Robert von Mayer (1814:1878) and Hermann Ludwig Ferdinand von Helmholtz (1821:1894) -- helped establish the "law of conservation of energy", which stated that in physical interactions energy was neither created nor destroyed, simply changed into different forms. This became the "first law of thermodynamics".
Their work was followed by that of the French physicist Nicholas Leonard Sadi Carnot (1796:1832), the English physicist William Thompson, later Lord Kelvin (1824:1907), and the German physicist Rudolf Julius Emanuel Clausius (1822:1888). They determined that heat energy can only spontaneously flow from hot to cold, not the reverse; that a temperature difference was required to allow an engine to extract work from heat; and that the greater the temperature difference, the greater the efficiency of the engine.
In 1850, Clausius came up with a subtle abstraction central to these concepts, defining the ratio of heat energy to temperature as "entropy". A given amount of heat energy at a high temperature had a lower entropy than the same amount of heat energy at a low temperature. Clausius showed that, through interactions in any isolated system, entropy had to increase, which was why heat flowed from hot to cold and engines required a temperature difference to operate.
Clausius' rule that entropy in a closed system must increase became known as the "second law of thermodynamics". Entropy is often casually defined as "disorder", but that is misleading. If we have a hot gas and a cool gas, we can obtain work from the temperature difference between them; if the hot and cool gases are mixed, then there is no more temperature difference and no work can be obtained from the system. Entropy actually refers to disorder of a certain specific sort, a "spreading out" or "dispersal" of energy, but it doesn't have much to do with more popular concepts of disorder, such as unmade beds or cluttered closets.
* While the physicists were nailing down the laws of thermodynamics, chemists were applying these developments to their own domain. In 1840, a Swiss-Russian chemist named Germain Henri Hess (1802:1850) published results of experiments that validated energy conservation in chemical reactions, showing that the final sum of heat produced or absorbed in a series of chemical reactions was the same no matter what different paths were used in the series. This became known as "Hess's Law", and established the branch of chemistry known as "thermochemistry".
In the 1860s, Pierre Berthelot took Hess's work a step further with the development of the "calorimeter", which was a chamber filled with water at a known temperature, with chemical reactions performed in a vessel inside the chamber. The amount of heat released by the chemical reaction could be precisely measured by the change in temperature of the water. Berthelot used his calorimiter to perform careful thermal analyses for a wide range of chemical reactions. A Danish chemist named Hans Peter Joergen Julius Thompsen (1826:1909) performed similar research in parallel.
Berthelot was particularly interested in why some chemical reactions were spontaneous, occurring simply if the reactants were put together, and others were not. He proposed that "exothermic" reactions, those that released energy, were spontaneous, while "endothermic" reactions, those that absorbed energy, were not. The idea was not so different from the concept that a ball will roll down a slope spontaneously, but has to be pushed back up the slope. The ball tends to seek its lowest energy level, and so, Berthelot claimed, did chemical reactions.
That was too simplistic. Some spontaneous reactions are endothermic; and there are some reactions that are "reversible", taking place simultaneously in both directions. Reversible reactions were known in the 1860s, having been examined in detail in 1850 by Alexander Williamson, the British chemist who had characterized the ethers. During his work on ethers, he noticed that a reaction of two reactants, say "A" and "B", that produced two products, say "Y" and "Z", would not necessarily run to completion, with the end result being a mixture of A, B, Y, & Z at seemingly fixed proportions. Williamson was shrewd enough to realize that the reaction of A & B to produce Y & Z hadn't simply stopped, that Y & Z were performing a reverse reaction to produce A & B, resulting in an equilibrium between the forward and reverse reactions:
A + B <--> Y + Z
This is just one example of a reversible reaction. Reversible reactions can have other forms, such as:
A + B + C <--> Z
-- but the reaction of A & B producing Y & Z will be used as a basic example here. This scenario would become known as "dynamic equilibrium", with Williamson becoming the effective founder of what is now called "chemical kinetics", the science of the rates of chemical reactions. Clearly, Berthelot's ideas needed some work.
In 1863, two Norwegian chemists, Cato Maximilian Guldberg (1836:1902) and Peter Waage (1833:1900), published a short document detailing their thoughts on the direction of spontaneous reactions. Take the general reversible reaction given above:
A + B <--> Y + Z
Suppose the initial proportions of A, B, Y, & Z are roughly comparable. Suppose also that the rate of the forward reaction of A & B to produce Y & Z is much higher than the rate of the reverse reaction of Y & Z to produce A & B. That means the forward reaction runs much faster than the reverse reaction, and the proportion of Y & Z in the mixture becomes larger while the proportion of A & B becomes smaller. The rising proportion of Y & Z increases the effect of the reverse reaction, while the falling proportion of A & B decreases the effect of the forward reaction.
By similar logic, adding more A or B will increase the rate of production of Y & Z, shifting the reaction "to the right" of the equation, and adding more Y or Z will increase the rate of production of A & B, shifting the reaction "to the left" of the equation. What Guldberg and Waage realized was that no matter how the reaction was manipulated, at equilibrium the ratio of all the products multiplied together to all the reactants multiplied together was a constant. If the concentrations of the reactants and products are denoted by square brackets ("[ ]") and the "equilibrium constant" is given by K, then:
[Y] * [Z]
----------- = K
[A] * [B]
Again, this is only for the simple example chemical equation given above:
A + B <--> Y + Z
For a more complicated chemical equation, such as:
2A + 3B <--> 3Y + Z
-- calculating the equilibrium constant gets correspondingly more complicated:
[Y] * [Y] * [Y] * [Z] [Y]^3 * [Z]
----------------------------- = --------------- = K
[A] * [A] * [B] * [B] * [B] [A]^2 * [B]^3
In any case, if the concentrations of products and reactants go out of balance, say by increasing the concentrations of one or more of them, the result will be a ratio greater than or less than K, which will then shift back to the equilibrium value of K.
Guldberg and Waage called their scenario the "law of mass action", simply because it only really considered the effects of concentrations -- masses of products or reactants -- on the balance of chemical reactions. Temperature of course was known to increase reaction rates and would have an influence on equilibrium, and so their scenario assumed that the reactions occurred at a constant temperature. Unfortunately, they published in Norwegian; few were aware of their work until it was translated into German in 1879.
BACK_TO_TOP* Across the Atlantic, in the United States, a physicist named Josiah Willard Gibbs (1839:1903) was working to apply the thermodynamics of Clausius to chemical reactions, publishing a series of papers in the late 1870s. What Gibbs realized was that a spontaneous chemical reaction was not just or even mainly dependent on the reaction releasing energy, it was also dependent on an increase in entropy. Gibbs devised a parameter known as "free energy" that was a function both of energy and entropy. Free energy is now more formally referred to in his honor as "Gibbs free energy" or just "G".
Gibbs was able to show in his work that if G increased in a chemical reaction, the reaction was spontaneous. Gibbs was painstakingly thorough and went much farther with the concept, showing how changes in concentration of a participant in a reaction could affect the free energy and so the rate of the reaction, providing a physical basis to the law of mass action. In Gibbs' terminology, changes in concentration of a participant changed the "chemical potential" of that substance, in a direct analogy to the potential energy of a mass in a gravitational field.
Finally, Gibbs was able to incorporate phase changes -- from solid to liquid, liquid to gas, and so on -- into his concepts, with his "phase rule", allowing him to predict the results of chemical reactions under a wide range of conditions. Gibbs laid the foundations of the field now known as "chemical thermodynamics", single-handedly defining most of its fundamentals. Although his name remains little known to the general public, he was one of the first world-class American theoretical scientists.
* Gibbs did not seriously address one of the major lingering issues in the physical nature of chemical reactions, the phenomenon of catalysis. As noted, decades before, Humphry Davy had shown how adding platinum powder to a chemical reaction could speed it up, without being consumed in the process. That seemed suspiciously like a "free lunch", and figuring out how it fit into chemical thermodynamics was a puzzle. Another one of the pioneers of physical chemistry was the Russo-German chemist Friedrich Wilhelm Ostwald (1853:1932), who wrote one of the first textbooks on the subject in 1887 and began the field's first journal. He was also an early European convert to Gibbs' ideas, translating Gibbs' papers on chemical thermodynamics into German in 1892, and giving considerable thought on how to fit catalysis into the system.
In a paper published in 1894, Ostwald suggested that catalysts could not change the nature of a reaction -- a reaction that wasn't spontaneous by itself wouldn't become spontaneous by the introduction of a catalyst -- but could speed up a reaction. He proposed that the catalyst bonded with one of the reactants, forming an intermediate compound that acted as a "template" where a second reactant could join with the first, releasing the catalyst to go through the cycle again. One common analogy of catalysis is of trying to write on a piece of paper without any surface to write on; it becomes much easier if a clipboard is available, though the clipboard really doesn't do anything to assist the writing process other than provide a "substrate".
The fact that the interaction of the catalyst with the reactants was short-lived explained why a small amount of catalyst could provide a seemingly disproportionate increase in reaction rate: the catalyst generated a reaction and then rapidly generated another, over and over again. Ostwald's model of catalysis remains the accepted wisdom today.
BACK_TO_TOP* Ostwald wasn't the only European chemist who helped promote Gibbs' chemical thermodynamics. A Dutch chemist named Hendrik Willem Bakhuis Roozeboom (1854:1907) promoted the Gibbs phase rule over the Continent. A French chemist named Henri Louis Le Chatelier (1850:1936) translated Gibbs' work into French in 1899; a decade earlier, Le Chatelier left his own mark on physical chemistry by proposing what would become known as "Le Chatelier's principle": Any change in the factors of a chemical system at equilibrium adjusts the system in such a way as to minimize the effect of the change.
In other words, raise the temperature, and the system will adjust itself to minimize the temperature change; increase the pressure, and the system will adjust to minimize the pressure change. This concept turned out to be neatly compatible with Gibbs' ideas.
Jacobus Van't Hoff, who discovered the tetrahedral structure of carbon bonding, independently duplicated much of Gibbs' work in the 1880s, helping hasten acceptance of Gibbs' writings once they finally reached a wider audience. Van't Hoff also made his own contributions to physical chemistry, in particular showing that solutes dispersed through a solvent in a fashion that could be modeled using much the same tools as used to describe the dispersal of gases.
A German chemist, Walter Hermann Nernst (1864:1941), applied the principles of chemical thermodynamics to electrochemistry, learning how to calculate the free energy changes due to the action of electrical storage cells. Nernst also performed pioneering research in chemical reactions that were promoted by light, helping to establish the field of "photochemistry", showing how sunlight could act as a catalyst in some classes of photochemical reactions.
Refined studies in electrochemistry also led to an improved understanding of the nature of ions. Studies of ionization had generally languished since Faraday's pioneering work early in the century -- though a German physicist named Johann Wilhelm Hittorf (1824:1914) did show in 1853 that some ions would propagate through a solution under the influence of an electric field faster than other ions.
Decades later, in 1887, a French chemist named Francois Marie Raoult (1830:1901) published the results of an intensive series of studies of solutions, establishing what became known as "Raoult's law", which requires a bit of explanation. The "vapor pressure" of a liquid is the pressure of the vapor produced by that liquid when it is placed in a vacuum chamber and molecules of the liquid evaporate into the evacuated space above the liquid. In a simple form, Raoult's law says that the vapor pressure of a solution will decrease as a nonvolatile solute is added to the solution. Water with sugar added will have a lower vapor pressure than pure water, and the vapor pressure will continue to fall as the concentration of sugar in the solution is increased.
This rule allowed Raoult to determine relative numbers of the particles of solute and solvent in a solution. One thing he noticed was that introducing a solute into a solvent depressed the freezing point of the solution, but the pattern by which it did so seemed puzzling. Raoult noticed that when nonelectrolytes were dissolved in a solution of water, the freezing point of the solution would drop by an amount directly proportional to the amount of nonelectrolyte. However, when an electrolyte like table salt was dissolved in water, the effect on freezing point was twice as great.
Raoult's law may seem like a bit of chemical fine print, but this quandary led to a major revelation when a Swedish chemist, Svante August Arrhenius (1859:1927) took a closer look at the situation. Arrhenius believed that the decline in freezing point was proportional to the number of particles, such as sugar molecules, dissolved in the solution. The fact that table salt had double the effect suggested further to him that, in dissolving in the water, salt split into its two components, sodium (Na) and chlorine (Cl), doubling the number of particles.
Following this same line of thought, Arrhenius realized that if the two components obtained an electric charge from splitting up -- say, sodium became positively charged ("Na+") and chlorine became negatively charged ("Cl-") -- they would support the conduction of electricity in an electrolytic cell. The positively charged Na+ ions would move to the negatively charged cathode, while the negatively charged Cl- ions would move to the positively charged anode.
Arrhenius proposed this idea, which he called "ionic dissociation", in 1884 as part of his doctoral thesis Since atoms were supposed to be indivisible, the examining board was skeptical; in their defense, Arrhenius's paper also left some things to be desired in terms of meeting proper standards. He was still awarded his doctorate, though he was given too low a rating to allow him to obtain an academic position. He didn't take the snub lying down, writing to prominent chemists to obtain support. The first of them he contacted ignored him, but finally he got in touch with Ostwald, who was enthusiastic and gave Arrhenius a position. Exactly how a table salt molecule could split into two charged parts didn't start to become clear for another 15 years.
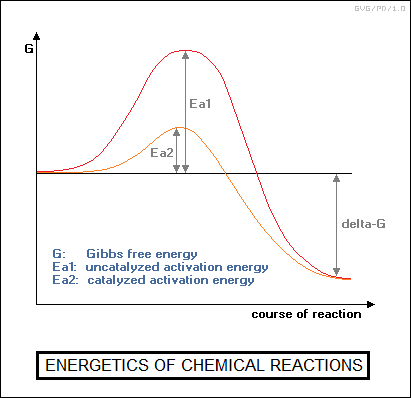
Arrhenius, one of chemistry's major figures, provided other insights. He suggested in 1889 that chemical reactions might need an "activation energy" to proceed -- just as a stone in a pit on top of a mountain needed to be pushed out of the pit before it could roll downhill. If this activation energy was low, its effect wouldn't be very noticeable, but if it was high, the reaction would not take place until, say, a certain temperature was reached -- and then it might take place abruptly. A mixture of hydrogen and oxygen gas seems innocent enough until the temperature reaches a certain level, and then the two gases combine explosively. Ostwald found this concept very handy in his considerations of catalysis, proposing that the complex formed between a catalyst and the reactants lowered the activation energy required for the reaction.
BACK_TO_TOP* Another branch of physical chemistry, the physics of gases, advanced rapidly in the 19th century. Early in the century, Gay-Lussac introduced two new gas laws that complemented Boyle's law (P1 * V1 = P2 * V2). The first was "Charles' law", which stated there was a neat inverse relationship between the volume and temperature of a gas at a constant pressure:
V1 / T1 = V2 / T2 -- or: V1 * T2 = V2 * T1
It had been devised sometime around 1787 by a French chemist named Jacques Charles (1746:1843), who hadn't published it; Charles, incidentally, invented the hydrogen balloon. The other was devised by Gay-Lussac and of course was known as "Gay-Lussac's law"; it postulated a similar inverse relationship between pressure and temperature, if the volume was constant:
P1 / T1 = P2 / T2 -- or: P1 * T2 = P2 * T1
It was straightforward to combine these three laws into a single "combined gas law", which stated:
pressure1 * volume1 pressure2 * volume2
___________________ = ___________________
temperature1 temperature2
This means:
As Newton had hinted well before with Boyle's law, the gas laws implied the existence of atoms. This implication was investigated by the great Scots physicist James Clerk (pronounced "Clark") Maxwell (1831:1879), who performed an analysis of the behavior of gases, applying statistical methods to the basic laws of mechanics to show how atomic theory could explain such things as Boyle's law. Similar efforts were performed in parallel by the Austrian physicist Ludwig Boltzmann (1844:1906).
The invention of "Maxwell-Boltzmann" statistics was a major advance in the physical sciences, adding the powerful methods of statistics to the toolkit, but it was also an idealized scheme, assuming "ideal gases" that consisted of point particles, with the point particles having no attractions to each other. It had been known earlier, through the work of the German-French chemist Henri-Victor Regnault (1810:1878), that gases deviated from Boyle's law at extremes of temperature and pressure, and so Boyle's law and the Maxwell-Boltzmann model needed some practical "tweaking" to conform to reality. The tweaking was performed by the Dutch physicists Johannes Diderik Van der Waals (1837:1923), who published an improved version of Boyle's law in 1873 that featured two constants, "a" and "b", that could be adjusted to factor in the size and mutual attractions of particles in a gas. The constants were different for each kind of gas.
As something more than a footnote to the combined gas law, it gradually became obvious that the heat energy of a system and the pressure-volume relation of that system were closely related. If we were to increase the heat energy of a gas in a closed system, if the volume didn't change, the pressure would increase. Were the volume to increase, the pressure would be reduced, with work being done by the expansion of the volume. That means simply calculating the heat energy in a system is missing something, and so it can be more useful to calculate "enthalpy" instead of energy, with enthalpy defined as:
enthalpy (H) = internal_energy (U) + P * V
The name is derived from the Greek, meaning "to put in heat". Although the idea was implicit in the work of Clausius and Gibbs, enthalpy was formally defined by the Dutch physicist Heiki Kammerlingh Onnes (1853:1926) in 1909.
* Another line of investigation focused on the liquefication of gases. Faraday, as mentioned, had worked on liquefying gases under pressure. He had found that some gases -- for example, oxygen, nitrogen, hydrogen, carbon monoxide, and methane -- couldn't be liquefied even under very high pressures. He suspected that it was impossible to liquefy them and called them "permanent gases". In the 1860s, however, an Irish chemist named Thomas Andrews (1813:1885) followed up Faraday's work. He liquefied carbon dioxide -- which Faraday had done -- and then started raising the temperature of the liquid. Andrews found that above 31 degrees Celsius, carbon dioxide could not be liquefied at any pressure, or more precisely the liquid and vapor state became very hard to sort out. In any case, in 1869 Andrews proposed that there was a "critical temperature" above which a gas could not be properly liquefied. That suggested that the "permanent gases" might well be liquefied if they were chilled below their critical temperature.
Joule and William Thompson had showed how the expansion of gases resulted in their cooling, and it wasn't much of a jump from this revelation to figure out a procedure to allow a gas to expand, cooling it; compressing it again in such a way as to keep from heating it up once more; and repeating this "refrigeration cycle" over and over again to produce lower and lower temperatures. In 1848, Thompson showed that this process couldn't be carried on to indefinitely lower temperatures, that there was an "absolute zero" temperature at -273.16 degrees Celsius. Nernst elaborated on this idea in a 1905 paper, showing that entropy would reach zero at absolute zero: all particle motion would cease. Nothing could be cooled below absolute zero, and in fact attempts to even approach this temperature ran into "diminishing returns", with each step towards absolute zero becoming more difficult and the curve of difficulty rising towards infinity as absolute zero was approached. This became known as the "third law of thermodynamics".
Thompson proposed a temperature scale in which the degrees had the same size as the Celsius scale, but in which the zero temperature was at absolute zero, meaning there would be no negative temperature values on this scale. This became known as the "Kelvin scale" in his honor, with the degrees given in "Kelvins (K)". In any case, using the new cooling techniques, during the 1870s a French physicist named Louis Paul Cailletet (1832:1913) and a Swiss chemist named Raoul Pictet (1846:1929) were able to liquefy oxygen, nitrogen, and carbon monoxide. Liquefying hydrogen proved more troublesome.
In the 1890s, a Scots chemist named James Dewar invented the "Dewar flask", a vessel intended for keeping materials cold. The Dewar flask had an outer and inner vessel, with the inner vessel silvered and a vacuum between the two vessels to reduce heat loss. Dewar used the flasks to store liquid oxygen and cool hydrogen gas, then went through refrigeration cycles to bring down the temperature of the hydrogen until it finally liquefied -- at 20 degrees Kelvin.
The inert gases discovered by Ramsay turned out to be even more difficult to liquefy. This was not surprising, since liquefication depends in part on the attraction of the particles of a gas to each other, and inert gases like helium don't interact very easily by their very nature. In 1908, Heiki Onnes used liquid hydrogen to chill helium, then went through refrigeration cycles to produce liquid helium -- at 4 degrees Kelvin.
BACK_TO_TOP* The following periodic table lists all the known elements and links to descriptive files for each element:
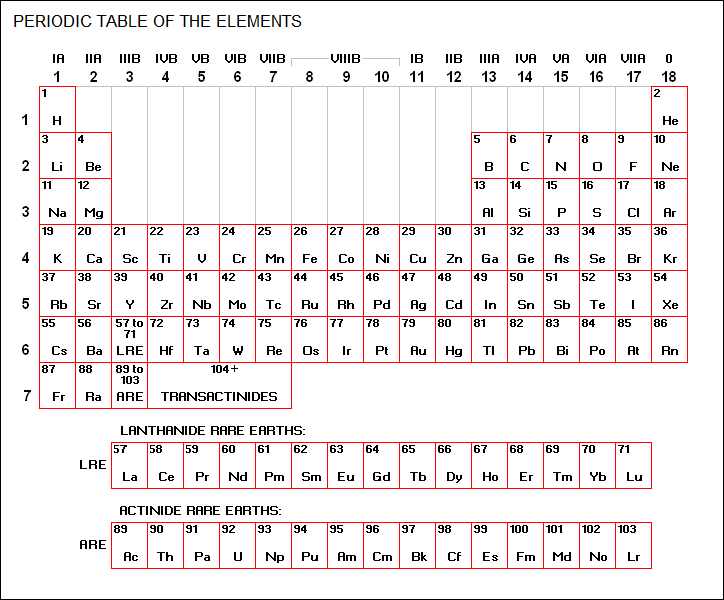
The entry for each element includes:
The format of the entries is flexible and varies a bit, for example to consolidate the noble gases, rare earths, and transactinide elements, or to account for the differing properties of allotropic forms. A general discussion follows the tables, outlining such items as (where relevant) names for ions of the element or groups based on the element, which often come up in names of chemical compounds; commercial sources of the element; applications of the element; and so on.
It should be noted that there are a number of alternative formats for the periodic table, some generated as proposals for a better format; some produced as an artistic or educational exercise; and some produced just for fun. Indeed, there is something of a cottage industry in the production of "periodic tables" for a wide range of topics, from desserts to fantasy elements to video game characters. In response to the proliferation of periodic tables, a periodic table was devised to organize all the periodic tables.
BACK_TO_TOP