
* The 19th century followed up Lavoisier's revolution by establishing the reality of atoms, with chemists identifying the different types of atoms and beginning to decipher their relative weights. Chemists would also begin to understand the interaction between electricity and chemistry, founding the science of "electrochemistry".

* In the course of their experiments, Lavoisier and many of his contemporaries had noticed that the reactants in their experiments seemed to combine in specific proportions: no matter how many times the experiment was conducted, the ratio of the reactants was always the same. A German chemist named Jeremias Benjamin Richter (1762:1807) performed work that helped validate this notion. Richter was investigating what are now known as "neutralization reactions", in which an acid is neutralized by mixing it with a complementary material known as a "base", producing water and a combined material known as a "salt". For example, hydrochloric acid (HCL) combines with sodium hydroxide (NaOH) to produce water (H2O) and table salt (NaCl). Richter noticed that the ratio of a particular acid to a particular base to produce a neutralization was always the same.
More formally, for any particular neutralization reaction, there was a fixed "equivalent weight" of an acid that neutralized a (generally different) equivalent weight of a base. For example, the ratio of the weight of sulfuric acid (H2SO4) and of sodium hydroxide needed to neutralize each other is 1.23, or in other words given a kilogram of sodium hydroxide, then the equivalent weight of sulfuric acid needed for a neutralization is 1.23 kilograms. Tables could be compiled of other equivalent weights of acids and bases. Richter published his results in 1792.
In 1799, the French chemist Joseph Louis Proust (1754:1826), working in exile in Spain, zeroed in on this concept by showing that copper carbonate contained fixed relative weights of copper, carbon, and oxygen in the proportions of 5:4:1 respectively. If he tried to form copper carbonate with an excess of copper, there would be copper left over; if he tried to form it with a deficit of copper, there would be carbon and oxygen left over. Proust went on to demonstrate similar sets of "definite proportions" for other materials.
One of Lavoisier's old associates, Claude Berthollet, insisted that Proust was wrong, that the proportions of materials involved in chemical reactions were not constant. In hindsight, although he was confused by bogus experimental results, Berthollet had good reasons for this belief, since his investigations had included analyses of certain types of glasses in which the ratio of constituents can vary within a range. He had no way of knowing that he had by chance picked materials that were unusual exceptions to the rules.
* An English Quaker schoolteacher named John Dalton (1766:1844) took the side of Proust, giving the "law of definite proportions" considerable thought and realizing that it implied the existence of atoms. Suppose, as an oversimplified example, that in a chemical reaction one atom of one element combines with one atom of another element to form a "product"; this is actually true in some cases, for example table salt (NaCl) or hydrochloric acid (HCl). Then the ratio by which the two atoms combine is also the ratio between the masses of the atoms themselves.

Dalton found that materials might actually combine in different reactions to give different proportions, but this could be explained if the atoms were combining in different ways. This became known as the "law of multiple proportions". Dalton used this law to determine rough relative masses of known elements, publishing an initial document in 1803 and following it up with a much more comprehensive work, titled A NEW SYSTEM OF CHEMICAL PHILOSOPHY, in 1808, in which he constructed a table of the elements, listing the relative weights of atoms.
Dalton's ideas were a big step forward, but there was only so far he could go with the data and tools at his disposal. For example, since two grams of hydrogen combined with eight grams of oxygen to form ten grams of water, he knew that oxygen was heavier than hydrogen. That was fine in itself, but the problem was that he had no idea of and no way of figuring out how many atoms of hydrogen combined with how many atoms of oxygen to form one molecule of water. He reasonably, though not correctly, assumed the ratio was 1:1, meaning that the molecular formula for water was "HO", and using that assumption determined that oxygen was eight times heavier than hydrogen.
Dalton did admit that as far as he knew, it could be 2:1, "H2O", which we now know to be the correct ratio, or some other ratio entirely. He also did not know that hydrogen and oxygen gas do not consist of solitary hydrogen and oxygen atoms, instead being composed of molecules consisting of twin atoms, "H2" and "O2". Dalton's table of elements and their atomic weights was littered with errors, but he was definitely on the right track, and over the next century his ideas would become the established wisdom.
BACK_TO_TOP* The revolution in chemistry during the 18th century had been paralleled by the rise of electrical science. The ancients of course had been aware of lightning and the small sparks generated by rubbing amber on fur or the like, but it wasn't until the English physicist William Gilbert (1540:1603) conducted methodical investigations of the generation of sparks by materials that the notion of "electricity", a word more or less coined by Gilbert from the Greek word for amber, started to become a science.
In 1733, a French chemist named Charles Francois de Cisternay du Fay (1698:1739) observed that rubbing of different classes of "electrical" materials gave them one of two kinds of electricity. Those with the same kind of electricity repelled each other, while those with opposite kinds of electricity attracted each other. Benjamin Franklin (1706:1790) -- America's first polymath of stature -- postulated that electricity was a fluid, what he called a "charge", which could either be in excess, resulting in one of the two kinds of electricity, or in deficit, resulting in the other. Connect objects with the different kinds of electricity together with a wire, and the fluid would flow as a "current" to level the balance of charge between them.
That would prove essentially correct, though Franklin did make a mistake that would be passed down from his time. Franklin could only guess which of the two classes of materials had an excess of charge, making them "positively charged", and which had the deficit of charge, making them "negatively charged". The odds were 50:50 on getting it right, and he guessed wrong, getting them backwards. Even today, thanks to Franklin's bad guess, electrical circuit analysis by convention assumes a flow of electrical current that's the opposite of the way current usually flows -- though surprisingly, this convention doesn't cause any real inconvenience.
Franklin's insight was still brilliant, and when he was assigned by the Continental Congress to be the ambassador to France, he was already known there as a world-famous scientist, the first scientist of stature to be produced by America. After Franklin, scientists began to learn more about electricity at a rapid rate, including discoveries about its relationship to and usefulness for chemistry.
In 1786, the Italian physiologist Luigi Galvani (1737:1798) noticed by chance that when he stuck a copper hook into the spinal cord of a frog, which was in turn hanging from an iron hook, the frog's legs twitched. Galvani performed experiments that showed other pairs of dissimilar metals caused similar effects. He felt that he was seeing the discharge of some sort of "animal electricity" from the frog's muscles. Such experiments became fashionable, and led to a popular belief that electricity was an elemental "life force". This belief was illustrated by Mary Shelley's Gothic horror novel FRANKENSTEIN, with the monster brought to life by electricity, and by a range of electrical quack medical equipment that remained popular into the 20th century. The "life force" concept still lingers in fantasy stories.
In 1800, an Italian physicist named Count Alessandro Volta (1745:1827) introduced the "voltaic pile", a stack of alternating zinc and silver disks separated by cloth patches soaked in acid that could produce an electric current. It was the very first chemical battery and the first predictable source of electric current available to scientists.
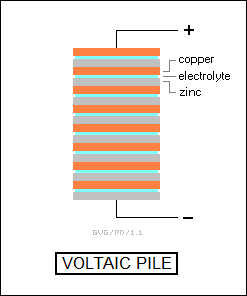
The electric current generated by the voltaic pile was obviously produced by some chemical reaction, though nobody had a clear idea of what. That ignorance didn't prevent other researchers from realizing that if electric current could be produced by a chemical reaction, then an electric current might also produce a chemical reaction. Within a matter of weeks of the publication of a description of the voltaic pile, two Englishmen, William Nicholson (1753:1815) and Anthony Carlisle (1768:1840), ran an electric current through water, and noticed that bubbles of gas formed on the plates of metal placed in the water to support the current from the pile. Oxygen formed on one plate, hydrogen on the other. Nicholson and Carlisle had used electricity to decompose a material, in this case water, into its constituents, hydrogen and oxygen. They noticed that the decomposition of water produced twice the volume of hydrogen as it did oxygen, even though the weight ratio was, as had already been known, 8:1.
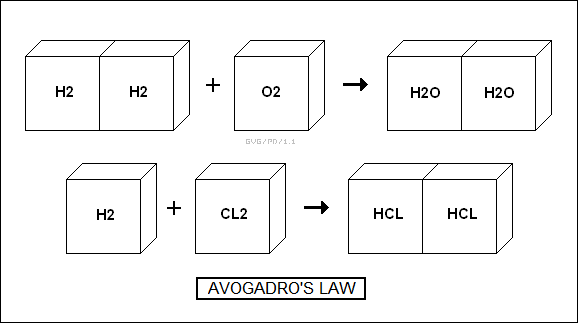
In 1808, a French chemist named Joseph Gay-Lussac (1778:1850) expanded on this notion by showing that two volumes of hydrogen combined with one volume of oxygen to produce water, and that similar experiments on other substances showed that the volumes of gases that entered into reactions did so in ratios of small integers. Gay-Lussac called this the "law of combining ratios". In 1811, an Italian scientist named Amedeo Avogadro (1776:1856) provided an explanation for this law, suggesting that at a fixed temperature and pressure, equal volumes of gas contained the same number of particles. If a party balloon is filled with hydrogen to the same pressure as a balloon full of oxygen, both balloons will contain the same number of particles, even though the balloon full of oxygen will be much heavier than the balloon full of hydrogen. Avogadro invented the term "molecule", incidentally, though there would be confusion between the notion of atoms and molecules for some time. Oxygen gas, for example, seemed to be made of atoms, not molecules, there being no way to know at the time and no reason to assume the particles were actually in the form of O2 molecules.
Avogadro's principle gave a way to compare the weights of the particles in the gas. As was known at the time, two volumes of hydrogen combined with one volume of oxygen to produce water, immediately suggesting that there were two hydrogen atoms for each oxygen atom. That didn't reveal that gaseous hydrogen and oxygen were actually diatomic molecules, but other experiments could provide hints. For example, chlorine gas also occurs as a diatomic molecule, Cl2, but when it's combined with diatomic hydrogen gas, H2, it forms two molecules of hydrogen chloride gas (HCl, what becomes hydrochloric acid in solution). A liter of chlorine gas and a liter of hydrogen gas combines to produce two liters of hydrogen chloride gas. Using a matrix of such experiments, chemists could obtain better estimates of relative atomic weights.
Unfortunately, Avogadro's ideas were lost in the noise for the time being, but chemists were still able to make progress in sorting out the elements. In 1818, a French chemist named Pierre Louis Dulong (1785:1838) and his colleague, French physicist Alexis Therese Petit (1791:1820), showed that the specific heat of elements seemed to be proportional to the atomic weight of those elements -- that is, if one element had twice the atomic weight of a second, it would take twice as much heat to raise the first element to the same temperature as the second. This is now known as the "Dulong-Petit law" or the "law of atomic heat".
In 1819, a German chemist named Eilhardt Mitscherlich (1794:1863) showed that two materials that crystallized on their own and could crystallize together were likely to have similar crystal structures, as if they were similar "parts" that could be assembled into the same arrangement. His "law of isomorphism" -- where "isomorph" means "same shape" -- meant that if the crystal structure of one material was known, then if a second material formed a crystal with the first, the second material could be assumed to have a similar crystal structure.
* Although the lack of recognition granted to Avogadro's principle meant that one of the most powerful tools for identifying relative atomic weights was neglected, the Swedish chemist Jons Jakob Berzelius (1779:1848), an exacting experimentalist, was able to use the other techniques available to him to perform a groundbreaking set of analysis over a period of two decades, finally publishing a table of atomic weights in 1828 that had a good close resemblance to modern tables of atomic weights. The work of Berzelius did much to establish Proust's law of definite proportions and Dalton's atomic theory as accepted fact, at least among chemists.
One of the problems with Dalton's atomic theory was that it listed so many elements, and that list was growing steadily larger. Berzelius' accomplishments included isolation of the new elements "cerium", "selenium", "silicon", "zirconium", and "thorium". Chemists were finding other elements as well:
Aristotle had got by on five elements -- earth, air, fire, water, aether -- and it seemed hard to believe that such a large number of elements were truly "elemental". In 1815, an English chemist named William Prout (1785:1850) proposed that they weren't: since atomic weights seemed to be nice neat multiples of the mass of the hydrogen atom, then atoms were likely to be agglomerations of hydrogen atoms. A century later, this idea would be seen as closer to the truth than Prout or any of his contemporaries had any way of knowing, but it wasn't supported by the data available at the time. For example, Berzelius' measurements of the atomic weight of oxygen showed that it actually had an atomic weight 15.9 times that of hydrogen. If oxygen was really made up of 16 hydrogen atoms, it should have an atomic weight exactly 16 times that of hydrogen. There was a reason for the discrepancy, but it wouldn't be understood until much later.
It should be noted here that Dalton and Berzelius used hydrogen as their reference value for atomic weights, assigning it a value of 1. However, as mentioned above, that gave oxygen an atomic weight of 15.9. Since so many compounds were oxides, incorporating oxygen, this discrepancy was cumbersome, and so a consensus was reached in the chemical community that oxygen be used as the reference instead, being assigned a value of 16. That gave hydrogen an atomic weight of about 1.008. The oxygen = 16 scheme would be retained as the basis for measuring atomic weights well into the 20th century, but it would run into difficulties of its own.
* Berzelius would have been remembered for his work on atomic weights, but that was by no means the full list of his accomplishments. In one of his most lasting contributions, he extended Lavoisier's modern scheme of chemical nomenclature by identifying elements by a letter or two -- for example:
__________________ hydrogen H helium He carbon C nitrogen N oxygen O sodium Na aluminum Al silicon Si phosphorus P sulfur S chlorine Cl potassium K calcium Ca iron Fe nickel Ni copper Cu zinc Zn silver Ag tin Sn gold Au mercury Hg lead Pb __________________
Some of the abbreviations were from Latin names for the element -- for example, in Latin iron is "ferrum", giving the name "Fe". Dalton had proposed a scheme in which different elements were listed as circles with different markings -- oxygen a simple circle, hydrogen a circle with a dot in it, carbon a blacked-out circle, and so on -- but much to his annoyance, Berzelius' scheme was the one that was accepted, largely it seems because it made life much easier for typesetters. This system of nomenclature would eventually be used over the entire planet, even in languages such as Chinese that aren't based on Roman characters.
In his prime, Berzelius touched on and influenced almost every aspect of his science. In his old age, he became a notorious scientific reactionary, obstinately refusing to accept new ideas until the evidence for them became completely undeniable. His influence faded out accordingly, though he was and remains respected for his earlier achievements.
* As far as Avogadro's principle went, it was revived in the 1860s by an influential Italian chemist named Stanislao Cannizzaro (1826:1910), and was finally accepted by the chemical community. Using Avogadro's principle, in 1865 a Belgian chemist named Jean-Servais Stas (1813:1891) released a new table of atomic weights that was another great step forward that wouldn't be bettered until the next century.
Although the system of atomic weights only gave the relative weights of atoms and molecules, chemists figured a scheme, known as the "gram molecular weight" or simply "mole", to translate it to actual weights in grams. The translating factor was simply the number of particles that measured out to the atomic weight, given in grams. This number was determined to be 6.02E23 molecules, which became known as "Avogadro's number", even though Avogadro hadn't suggested the value himself. For example, the gram molecular weight of O2 oxygen gas was 32 grams; a mole of H2 gas was about two grams, and the gram molecular weight of water, H2O, was about 18 grams. This system of measure made accounting for reactants and products in chemical reactions much simpler, helping sweep away the confusion that had plagued chemists for the previous few decades.
BACK_TO_TOP* The use of electricity by Nicholson and Carlisle led to the use of electricity to isolate new elements. An English chemist named Humphry Davy (1778:1829) built an enormous bank of batteries to try to break down materials that seemed to be compounds but resisted decomposition by conventional chemical schemes. Davy originally tried to decompose such materials by dissolving them in water and then running a current through the water, but all that accomplished was to decompose the water into hydrogen and oxygen.
Obviously using a solution was no good, so he decided to melt the suspicious materials and then run an electric current through the hot melt. In 1807, he ran a current through the mineral potash (potassium carbonate or K2CO3) and obtained little globules of a metallic material, which he named "potassium". A week later, Davy performed the same trick with the mineral soda (sodium bicarbonate or Na2CO3) and isolated another metal, which he named "sodium". Both potassium and sodium were highly reactive: a pellet of either thrown onto water would burst into flame and skitter about excitedly on top of the water. No wonder they were so hard to isolate -- anything that combined with other elements so eagerly was obviously hard to pry apart from them again.
Berzelius proposed some improvements in the Davy's scheme, and the next year, 1808, Davy used the improved technique to extract "magnesium" from magnesia (magnesium oxide or MgO); "strontium" from strontia (now known as strontianite, more formally strontium carbonate or SrCO3); "barium" from baryta (barium sulfate or BaSO4); and "calcium" from lime (calcium oxide or CaO). Davy had single-handedly expanded the list of elements by a good fraction.
* Davy's isolation of these elements would have been enough to have ensured his name in the history of chemistry, but like Berzelius, Davy produced a long list of achievements. He was one of the first to demonstrate that chlorine gas (Cl2), which had been produced by Scheele decades earlier, was actually elemental; incidentally, chlorine gas is dangerously toxic and it may have been one of the contributing factors to Scheele's early death, though Davy was apparently cautious in dealing with the nasty greenish vapor. Davy also showed that hydrochloric acid (HCl) doesn't contain oxygen, puncturing Lavoisier's theory that all acids were oxides. In 1816, in one significant finding, Davy found that adding platinum powder could increase the rate of chemical reactions, without the powder being consumed by the reaction. Materials with such properties were given the name of "catalysts" by Berzelius in 1835, with their effect in chemical reaction referred to as "catalysis".
However, Davy's most important discovery was of a young man named Michael Faraday (1791:1867). Faraday had been born into grinding poverty; when he was apprenticed to a bookbinder, he began to read the books, with his employer's encouragement. After reading a popular text on chemistry for women, he finally landed a job as Davy's lab assistant and, on occasion, personal servant. From such humble beginnings, Faraday became one of the greatest experimental scientists in history.
Faraday's achievements covered a wide range of ground, but he is mostly remembered for his comprehensive studies of electricity and magnetism, in the course of which he came up with the concept of a "field" by observing the patterns given by iron powder on top of a sheet of paper laid on top of a magnet. The field provided a fundamental tool for the modern understanding of forces.
Faraday also performed careful studies of electrochemical processes, establishing, with the help of an English scholar named William Whewell (1794:1866), the basic modern nomenclature for an electrochemical system: the electrical decomposition of a material became known as "electrolysis", while a solution or melt that could carry a current became known as an "electrolyte" (making one that didn't a "nonelectrolyte"), and the two metal plates became known as "electrodes". The negatively charged electrode was called the "cathode", while the positively charged electrode was called the "anode". In 1832, Faraday went on to publish his "two laws of electrolysis":
Some chemists suspected that these laws implied that electricity was provided in some sort of unit. Faraday was murky on the actual mechanisms. He believed that the electric current was carried through the solution or melt by particles that he called "ions", from the Greek word for "wanderer". Ions that migrated to the cathode were called "cations" (pronounced "cat-ions", not "cashions") and those that migrated to the anode were called "anions" (pronounced "an-ions", not "anyons"). He had no concept of their real nature, and for the moment that was as far as things went.
Faraday's other contributions to chemistry included liquefying a number of gases, including chlorine and sulfur dioxide, using cooling under pressure. He figured out a way to obtain very low temperatures, by the standards of the time, by pressurizing a gas to liquid form, allowing the liquid to cool to ambient temperature, and then releasing the pressure. The evaporation of the gas caused a steep increment of cooling, and with such techniques Faraday was able to reach temperatures as low as -78 degrees Celsius.
While some patrons become very proud of the achievements of their proteges, Davy felt increasing resentment towards Faraday as the younger man's fame grew. Davy went so far as to accuse Faraday of plagiarizing the prominent William Wollaston, even though Wollaston himself denied it and praised Faraday's work. Paintings of Davy show a man dressed in the height of Regency fashion, giving an impression of vanity, born out by his conduct. In fact, Davy was something of a media star, with his lectures and demonstrations in high demand, and his avenues of research were selected at least partly on the basis of their flashiness and appeal to the public. He took a high-profile celebrity wife, though he ended up regretting it.
In contrast, Faraday was a devout, gentle man, a member of an undemonstrative nonconformist Christian sect, photographs showing him with plain Victorian dress and tousled hair. He did little to deliberately court attention, though as his fame spread, he got it whether he liked it or not. Although Davy was brilliant and ingenious, so was Faraday, and Faraday's meticulous methodicalness ensured that he would eventually reach a stature much greater than that of his mentor. Davy could not always hide his resentment, though Faraday did his best to be patient; after all, Davy had been his mentor and had opened doors for Faraday that would have been otherwise closed to him.
BACK_TO_TOP* The development of atomic theory, the determination of atomic weights, and the concept of gram molecular weight gave chemists tools for a precise quantitative approach to chemistry. Although there was confusion over molecular structures in the era of Davy and Berzelius, eventually chemists would be able to reduce chemical reactions to neat "chemical equations". In modern times, chemical equations would become painfully familiar to every high-school chemistry student.
For example, sulfuric acid (H2SO4) and sodium hydroxide (NaOH) react to form water (H2O) and sodium sulfate (Na2SO4). This chemical equation can be expressed as follows:
H2SO4 + NaOH --> H2O + Na2SO4
The "reactants" or "reagents" are on the right side of the equation, with the "products" on the left. The problem with this equation is that the numbers of atoms in the reagents don't balance with the numbers of atoms in the products. Breaking the equation down into individual atoms gives:
3 x H + 5 x O + S + Na --> 2 x H + 5 x O + S + 2 x Na
The trick of course is that more than one molecule of each reagent or product may be involved in the chemical reaction. Sorting out the proper numbers is like working out a simple puzzle: since there are two sodiums on the right side of the equation but only one on the left, then it would seem useful to start by thinking two sodium hydroxide molecules are involved:
H2SO4 + 2NaOH --> H2O + Na2SO4
This gives:
4 x H + 6 x O + S + 2 x Na --> 2 x H + 5 x O + S + 2 x Na
That doesn't balance out either, with the left side of the equation having two hydrogen atoms and one oxygen atom more than the right side. Conveniently, these three atoms make up one water molecule, so the final balanced equation is:
H2SO4 + 2NaOH --> 2H2O + Na2SO4
This balanced equation gives the precise relationship between the reactants and products. Actually determining the ratio of masses requires the use of molar weights. Given the approximate atomic weights of H = 1, O = 16, Na = 23, and S = 32, the molar weights for these elements are simply 1, 16, 23, and 32 grams respectively, and the molar weights of the molecules in the equation above are:
H2SO4: 2 x 1 + 32 + 4 x 16 = 98 grams NaOH: 23 + 16 + 1 = 40 grams H2O: 2 x 1 + 16 = 18 grams Na2SO4: 2 x 23 + 32 + 4 x 16 = 142 grams
According to the balanced equation, one mole of sulfuric acid / H2SO4 (98 grams) and two moles of sodium hydroxide / NaOH (2 x 40 = 80 grams) yields two moles of water / H2O (2 x 18 = 36 grams) and one mole of sodium sulfate / Na2SO4 (142 grams). That gives 98 + 80 = 178 grams of reagents and 35 + 142 grams = 178 grams of products, and the equation balances. Incidentally, performing the calculation using more exact atomic weights -- H = 1.008, O = 16, Na = 22.99, S = 32.06 -- gives 178.07 grams on both sides of the equation.
Chemical reactions can in many cases proceed in two directions, indicated in chemical equations using a double-ended "two-way" arrow. Chemical equations may also include annotations, such a temperature over the arrow to indicate the conditions under which the reaction will occur, or comments that a reactant or product is a solid (s), liquid (l), gas (g), or exists as a solution in water (aq / aqueous).
* Of course, the concept of a mole comes in useful for the description of solutions. Solutions are defined by the "concentration" in percent of the solute in the solvent. A 15% solution of ethanol, a liquid, in water is defined as 15 volumes of ethanol in 85 volumes of water, while a 15% solution of table salt, sodium chloride, in water is defined as 15 moles of salt in 85 moles of water. If the concentration is very low, it is instead given as "parts per million (PPM)" or "parts per billion (PPB)".
BACK_TO_TOP