
* The concept of mechanical waves leads to the concept of light, which is also a wave phenomenon. However, its nature is somewhat more devious than sound waves and other mechanical waves, and in fact has some maddeningly contradictory aspects that eventually broke through the boundaries of classical physics. This chapter provides a description of the behavior of light.
* The concept of light is as fundamental as that of matter and predates recorded history. Obviously there was something present during the daytime that allowed people to see that wasn't around at night, and that light was emitted by hot objects such as the Sun, or by fires. It was also obvious that light carried energy, since it brought warmth; that it came in the different colors of the rainbow; and that it could be reflected by shiny surfaces, or refracted by passing through water or crystals.
The ancient Greeks had some confused ideas about light, such as failing to understand that the eye operated by passively receiving the light that fell on it, instead believing the eye actually generated the light. They still got some things right. The philosopher Empedocles (~490:430 BCE) speculated that light had a finite speed, and Aristotle correctly linked the occurrence of rainbows to the reflection of light from raindrops, though he had no real idea of why the result was a colored band. The mathematician Euclid (around 300 BCE) correctly understood the rules of reflection of mirrors.
The confusion over the operation of the eye was finally resolved through experiments performed by the Egyptian scientist Ibn Al Haythen, known in Europe as "Alhazen" (~965:1038). However, the modern understanding of optics did not begin until the 17th century, as researchers began to determine the precise rules of reflection and refraction, leading to the introduction of optical equipment, such as eyeglasses, microscopes, telescopes, and cameras.
* Following the beginnings of modern optics, scientists debated for over a century whether light was a particle or wave phenomenon. The particle theory had the advantage of not requiring any medium for the propagation of light, since there didn't seem to be any medium there to carry it, but the wave theory was far superior in explaining phenomena such as refraction. In the 17th century, this debate was carried on by Isaac Newton, who advocated the particle theory, and the Dutch scientist Christiaan Huygens (1629:1695), who advocated the wave theory.
In 1803, Thomas Young -- the inventor of Young's modulus -- published studies of the behavior of a light source when passed through two narrow slits. If light were a particulate phenomenon, the light falling on a surface beyond the slits should have been concentrated directly behind the slits, but what he observed was a pattern of alternating light and dark lines, the product of wave interference. In hindsight, that was enough to tip the scales toward the wave theory of light, but such was the authority of Newton that it took another 20 years for people to come around.
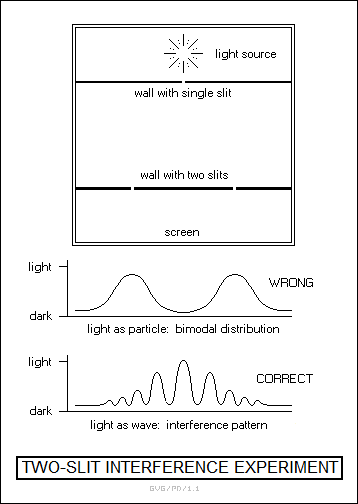
The clincher to the wave theory was provided by the French physicist Augustin Jean Fresnel (1788:1827), who performed an analysis that showed that the shadow cast by a small solid sphere in a bright light should have a bright center. This was due to the fact that light diffracted around the edges of the sphere, which wouldn't have happened if light had been a particle. Fresnel performed this analysis to a board of inquiry that didn't want to believe it, but the board found his evidence irrefutable. That seemed to resolve the debate in favor of the wave theory, though it still left open the problem of the medium that light used for propagation.
Of course, Newton was perfectly aware of light interference effects, having devised an experiment known as "Newton's rings". This involves placing a glass lens, curved side down, on a mirror. Light passing through the lens will be partly reflected at its bottom face, with the rest reflected from the mirror, with the two components interfering to create a set of concentric rings. Newton knew this was a wave phenomenon, but suggested that it was due to some wave set up by the passage of light particles through the glass. In terms of 20th-century physics, Newton was in some ways on the right track to the truth, but that is another story.
* It is very easy to observe light interference at home. Dexter could do so by holding up a hand with his fingers pressed together, and then observing a bright light through the cracks between his fingers. He would see one or more fine black hairlines in the crack, due to light interfering as it passed around the edges of his fingers.
Optical interference effects have practical uses. They are the basis of "interferometers", highly sensitive instruments in which two light beams sent through alternate paths are allowed to interfere, with any slight change in the relative paths of the two beams causing alternations in the intensity of light output. An interferometer can be used as an "optical gyroscope" by arranging the two light paths as alternate routes from one vertex of a square to the opposite vertex, with the rotation of the square around its center causing the effective path of light along one side to shorten and around the other side to lengthen. Another scheme is to fit some class of sensor into one of the paths, with the sensor's optical properties changing with the parameter to be measured.
Interference effects are also used in "holography", with interference patterns from the reflections of light off an object recorded in photographic film as a "hologram". Shining light through the hologram will reconstruct the image in three dimensions, and the image will seem to float in space.
* A half century after Thomas Young, James Clerk Maxwell conducted a brilliant theoretical analysis of electric and magnetic fields, concluding, among other things, that these fields could propagate through space in a coupled fashion as a wave. Maxwell's analysis not only identified light as such an "electromagnetic wave", but also predicted that such electromagnetic waves would exist over a very wide range of frequencies, most of which were invisible to the eye.
Scientists had known for decades there were invisible forms of light. Early in the 19th century, the Anglo-German astronomer Sir William Herschel (1738:1822) used a prism to break sunlight up into its rainbow spectrum, and then moved a thermometer through the spectrum to see how much warmth it contained. The temperature rose steadily from the blue end of the spectrum to the red end of the spectrum, and then continued to rise as Herschel moved the thermometer past the red end of the spectrum. This invisible form of light was named "infrared" light.
Maxwell's analysis extended this concept by showing that electromagnetic radiation existed over an infinite range of frequencies, and that only a limited range of these wavelengths were visible to the eye. Visible light quickly became only a small subset of the entire "electromagnetic spectrum". Long wavelength electromagnetic radiation became identified as "radio waves" that could be generated and detected with electronic gear, leading to the foundation of radio communications.
* One of the questions that arose with early investigations of light was whether it had a finite speed. Galileo and an assistant performed some experiments, shining lanterns at each other from distant hills, but all he was able to conclude from such a clumsy scheme was that if light had a finite speed, it was very fast.
In 1676, the Danish astronomer Olaus Roemer (1644:1710) noticed that the predictable timing of the eclipses of the moons of Jupiter shifted relative to the position of the Earth, advancing when the Earth was on the same side of the Sun as Jupiter and falling back when the two planets were on opposite sides of the Sun. He was able to obtain an estimate of the speed of light by measuring the delay and using estimates of the size of Earth's and Jupiter's orbits; his estimate was that light traveled at about 227,000 kilometers per second. That was about 75% of the real value -- not on the mark but well in the ballpark, and an impressive accomplishment given the tools available to him.
In the 19th century, improved technologies permitted a much more accurate measurement of the speed of light. The French experimentalist Armand Hippolyte Louis Fizeau (1819:1896) used a system of rotating toothed wheels, intercepting a light beam that was routed between them over a roundabout path with a set of mirrors to increase the path length. The speed of rotation of the wheels was increased until the light was blocked, allowing calculation of the speed of light from the path length, the size of the gap between the teeth of the wheels, and the rate of rotation. His colleague Jean Bernard Leon Foucault (1819:1868), best known for his invention of the Foucault pendulum, performed even better measurements using a system that was based on spinning mirror drums instead of toothed disks. Foucault's value turned out to be very close to 300,000 kilometers per second.
BACK_TO_TOP* Electromagnetic (EM) radiation propagates through a vacuum as a wave moving at (very close to) 300,000,000 meters per second. This gives the relationship between frequency and wavelength:
300,000,000
wavelength = -----------
frequency
EM radiation occurs over a continuous spectrum of wavelengths, ranging from low-energy long wavelengths at the bottom of the spectrum to high-energy short wavelengths at the top of the spectrum. The visible light that humans can sense is only a very small part of the full EM spectrum. The spectrum extends through longer wavelengths, down through the "infrared", "microwave", and "radio" regions; and through shorter wavelengths, including the "ultraviolet", "X-ray", and "gamma-ray" regions.
THE EM SPECTRUM __________________________________________________________________ radio 30 centimeters or longer microwave 1 millimeter to 30 centimeters infrared 0.77 microns to 1 millimeter visible 0.39 to 0.77 microns ultraviolet 10 nanometers to 0.39 microns X-ray 10 picometers to 10 nanometers gamma ray less than 10 picometers __________________________________________________________________ A "micron" is a micrometer, or millionth of a meter. There is actually overlap in the definitions of all the bands except the visible band, and the figures given here are useful simplifications.
The energy of EM radiation increases with frequency. Radio waves and microwaves aren't very energetic, though as a microwave oven demonstrates they can still transfer a lot of energy if produced in quantity. High-frequency ultraviolet waves, X-rays, and gamma rays are very energetic, but fortunately the ozone layer blocks most of this high-energy radiation and prevents it from doing us harm.
The visible-light region of the spectrum is divided into "color sub-bands". These range from low-energy red and orange bands, to more energetic and longer-wavelength yellow and green bands, to high-energy blue and violet bands. Light emitted by the Sun and by incandescent light bulbs consists of a mix of these bands, and is known as "white light".
COLOR SUBBANDS __________________________________________________________________ red visible 0.622 to 0.770 microns orange visible 0.597 to 0.622 microns yellow visible 0.577 to 0.597 microns green visible 0.402 to 0.577 microns blue visible 0.455 to 0.402 microns violet visible 0.390 to 0.455 microns __________________________________________________________________
Isaac Newton was one of the first to list the colors of the visible light spectrum. He defined seven colors, not six, inserting "indigo" between blue and violet. Few could ever make it out as anything more than dark blue, and apparently the only reason he used it was because he considered the number "7" as having mystical significance.
BACK_TO_TOP* Light may be emitted by matter when it is energized. One of the most common ways in which light is emitted occurs when a solid, liquid, or gas is heated, a process that is known as "incandescence".
The light from an incandescent solid or liquid object is emitted in a continuous range of frequencies, or "continuous spectrum". The continuous spectrum emitted by a solid or liquid object generally approximates that produced by a "perfect emitter" or "blackbody". This is generally modeled as a hollow sphere with a hole in it, with the radiation produced in the interior of the sphere observed through the hole. For this reason, blackbody radiation is also sometimes called "cavity radiation".
In a blackbody, the intensity of the light across the frequency spectrum rises to a peak corresponding to a specific temperature, and then declines rapidly for higher frequencies. As a blackbody is heated and grows hotter, it first emits in the infrared, then glows dull red, next turning orange as it gets hotter, then yellow, then white, and finally bluish-white, with the colors corresponding to increasing peak frequencies. As the blackbody is heated even more, emission begins to occur in the ultraviolet, then in the X-ray region and finally the gamma-ray region of the spectrum.
The blackbody would still appear to be bluish-white to the eye, though anyone close enough to inspect it once it got that hot would be much safer someplace else. The total energy output of the blackbody increases at approximately the fourth power to temperature, meaning that if the temperature is doubled, the energy output is increased by sixteen (2^4) times.
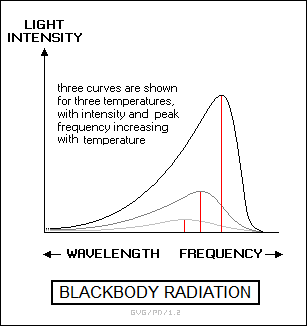
Stars are reasonable if not perfect approximations of blackbody emitters. Relatively cool stars are red, while very hot stars are bluish white. Small cool red stars have relatively low energy output and live for a very long time, while large hot stars use up their energy rapidly and so have short lives. A more mundane example of thermal emission is the old, now increasingly obsolete, incandescent light bulb, which glows by heating a filament with an electric current.
Most of the light emission from objects in our surroundings is in the infrared. This emission allows infrared scopes and cameras to detect objects in the dark. The appearance of objects seen from their thermal emission can be confusing since it is very different from that seen in daylight, and FLIR imagers often have a switch to flip the scene from "white hot" to "black hot" to help a user figure out a scene.
* Incandescence in gas works somewhat differently than it does in solids and liquids. Changes in the energy state of electrons in gaseous atoms and molecules cause a hot gas to emit electromagnetic radiation only at certain frequencies that are specific to the gas. This pattern is known as the "line emission spectrum" of the gas. Gas-filled lamps used in front-window displays of bars and other commercial establishments are filled with gases excited by an electric current, with the color determined by the type of gas. Neon, for example, gives an orange light.
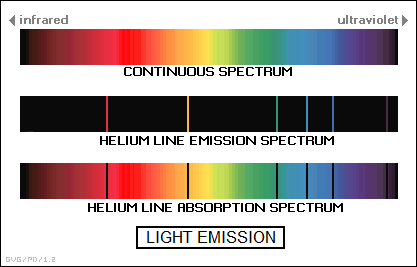
Interestingly, if white light is shined through a cool gas, the gas will absorb light at these specific frequencies, forming a "line absorption spectrum" that consists of dark lines across the rainbow spectrum in the background. The specific pattern of lines in an emission or absorption spectrum can be used to determine the chemical composition of everything from atmospheric gases to distant stars. This technique is known as "spectroscopy".
The basic concept was proposed in 1823 by Sir John Herschel (1792:1871), the son of Sir William Herschel, and the details were fleshed out by two German researchers, Robert Wilhelm Bunsen (1811:1899), and Gustav Robert Kirchhoff (1824:1887). They collaborated to develop the first formal "spectroscope" to observe line emission spectra and also characterized the line spectra of many gases. The basic rules of line spectroscopy, as described above, are known as "Kirchhoff's laws".
Such line spectra can also be used to determine the velocity of an object along our line of sight (known as its "radial velocity"). As with sound waves, light can be Doppler-shifted, and the entire line spectrum of a cosmic object will shift in frequency by an amount proportional to its radial velocity. If an object is coming towards us, the frequencies will be higher, or "blueshifted"; and if it is going away from us, the frequencies will be lower, or "redshifted".
* There are also "luminescent" light emission processes. The best known is the "arc discharge" due to a lightning bolt, which ionizes the air and causes the electrons to emit light. For another example, if some substances are exposed to high energy light, they may emit light at a lower energy, a process known as "fluorescence". In a fluorescent light tube, an electric current flowing through a mercury gas produces a wide range of wavelengths, which then excite a "phosphor" material coating the inside of the tube to emit visible light. Some of these substances will continue to emit light in a fading fashion after the energy source is turned off, a phenomenon known as "phosphorescence".
Chemical and biochemical processes can emit light as well. One of the more interesting modern examples is the "light stick" toy, which when twisted mixes two chemicals that react and emit pale light, a process known as "chemiluminescence". Many biological organisms also emit light by chemical processes, though the emission is called "bioluminescence" in this case. Fireflies are the best-known example, though sailors who have passed through tropical seas are also well aware of marine microorganisms that will emit light when disturbed, leaving a glowing trail as the bow of a ship plows through the water.
There are "triboluminescent" processes that involve light emission due to stresses on a material. This can be easily demonstrated by giving a plastic tray of icecubes a quick strong twist in a dark room: it will emit a faint flash of pale blue light. Intense sound waves can also create light emission in certain materials, a process known as "sonoluminescence".
* Modern lasers involve light-emission phenomena that aren't completely out of the domain of classical physics. A gas laser is basically a gas-filled lamp built as an optical resonant cavity, with a mirror at one end and a partly-transparent mirror at the output end and the length of the cavity being some integer multiple of one of the wavelengths of line emission for the gas. Energizing the gas-filled lamp causes light to build up in the resonant cavity and leak out the partly transparent mirror as a tightly focused beam.
Some classes of solid-state lasers substitute a fluorescent material -- such as a rod of ruby crystal -- for a gas-filled tube; not incidentally, fluorescence is a line-emission process. However, the solid-state lasers like those used in CD or DVD players are a concept that would seem like complete magic to a classical physicist, and so have to be discussed elsewhere.
BACK_TO_TOP* When light strikes matter, it can be absorbed, reflected, or passed through. A black plush cloth will absorb most of the light; a mirror will reflect most of the light; and a sheet of clear glass will allow most of the light to pass through.
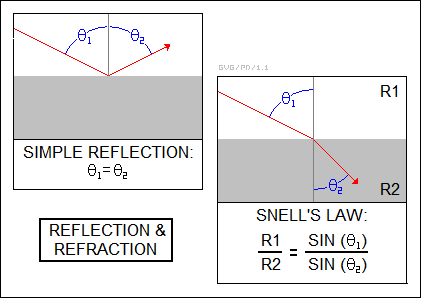
Reflection is a simple process. If a beam of light strikes a smooth, flat reflective surface such as a mirror at an angle, it will bounce off the mirror at the same angle as it struck. Of course, if the surface is rough, it will be scattered instead of neatly reflected. The angle of reflection does not change with the wavelength of the light.
Light passing through a transparent material tends to be refracted, with the amount of bending depending on the material's "index of refraction", which is given by:
speed_of_light_in_vacuum
index_of_refraction = ----------------------------
speed_of_light_in_material
The index of refraction is close to 1 in air, about 1.3 in water, about 1.5 in glass, and about 2.4 in diamond. Refraction can be seen by observing from certain angles a spoon stuck in a glass of water, which appears bent under the water. I have a novelty lamp in the form of a twisted, water-filled vertical tube that produces some really spectacular refractive effects.

A precise index of refraction can in general be only given for a single wavelength of light, since it varies with wavelength. This is why a prism creates a tiny "rainbow" when sunlight shines through it, since the different wavelengths of light bend in a range of different directions. This process is known as "chromatic dispersion".
The amount of bending for a material with a given refractive index is given by "Snell's Law", after the Dutch mathematician Willebrord Snellius or Snell (1580:1626), which is very important for the design of lenses for optical instruments. If a beam of light is passing through a medium with refractive index R1 and strikes the surface of a medium with a refractive index R2, then the angle of the beam relative to the vertical in the two mediums is given by:
_________________________________________________________________________ R1 / R2 = SIN( angle1 ) / SIN( angle2 ) _________________________________________________________________________
-- where SIN is the trigonometric sine function.
Reflection and refraction are the cause of rainbows. If Dexter has the Sun to the back of his head and observes a rainstorm, light from the Sun passing through the rain droplets is partially reflected and bounced back towards him, experiencing refraction and chromatic dispersion as it passes through the droplet. The rainbow will appear at an angle of about 40 degrees around the line established by the Sun and Dexter's head, though of course the angle varies a bit with color.

Remnants of the sunlight will bounce around through the droplet again, and if the sunlight is bright enough and the rain dense enough, Dexter will see a faint "double rainbow", at about 50 degrees from the line from the Sun through his head. It is sometimes even possible to see a short and faint section of a triple rainbow, but it is very rare. Another unusual occurrence, though not quite as rare, is a "halo" that appears around the Sun on very humid and hazy days, which is caused by sunlight passing directly through water droplets and being refracted into its spectral components.
Refraction is the cause of desert mirages. Cold air has a higher index of refraction than warm air, and the air close to the hot desert sands is hotter than the air above it. The temperature gradient through the air causes light to curve upward, with the result that distant objects seem to "float" upward, which can cause mis-estimations of distances and other confusion.
Incidentally, if light strikes a medium of different refractive index at a shallow enough angle, all the light will be reflected. This phenomenon is known as "total internal reflection". One application of total internal reflection is "optical fibers", the threads of glass sometimes used in novelties and for optical data-communications links. These optical fibers have a glass core with one index of refraction and an outer layer or "cladding" of another, causing light sent down the axis of the fiber to be reflected down its length with few losses.
As mentioned earlier, light is also diffracted, bending around the edges of objects. Diffraction, like refraction, is wavelength dependent, and can produce rainbows from a light source. Astronomers performing spectroscopy of stars and other cosmic objects normally do not use a prism to obtain the line spectra of those objects, since a prism would have a narrow field of view. Instead, they use a "diffraction grating", which is essentially a grooved mirror surface, with the reflected light diffracting around the edges of the grooves. A good home-grown equivalent of a diffraction grating is a CD or DVD disc: if held to the light it displays pretty colors, due to diffraction from the disc tracks.

* The actual colors we perceive in the real world arise from a number of processes. Thermal emitters like the Sun and line emitters such as neon lamps produce their light directly, but all other objects produce their light by reflection.
Objects with light-colored surfaces reflect most of the light falling on them, while those with dark-colored surfaces absorb most of the radiation falling on them, with the energy then released as thermal emission. Some objects may selectively absorb certain wavelengths and reflect others. Plants absorb blue and red wavelengths to perform photosynthesis, while reflecting green wavelengths, which is why most plants appear green.
The reflectivity of an object is also dependent on its smoothness. A very smooth surface reflects light without much scattering, and is known as a "specular" or "mirror" reflector. Calm bodies of water are specular reflectors. A rough surface scatters light, and is known as a "diffuse" reflector.
Except for the few of us who have been astronauts, we observe light that has passed through the atmosphere. The atmosphere not only blocks light through hazes and clouds, but also selectively scatters light at the shorter wavelengths. Scattering from the molecules in the atmosphere diffuses short-wavelength blue light from the Sun, causing it to appear all over the sky and so making the sky blue. On the other side of the coin, this same scattering of blue light makes sunsets tend to be red: the shorter "bluish" wavelengths of light are scattered, subtracting them from the Sun's light and leaving longer "reddish" wavelengths behind. Smoke from forest fires or heavy industrial pollution dumps large sooty particulates into the air, which cause even greater reddening, resulting in dramatic "blood red" sunsets.

Incidentally, it is only possible to see a light beam from the side because of scattering. The well-known pyramidal Luxor Hotel in Las Vegas has searchlights in its apex shining up into the sky, which the designers believed would cap the building with a spectacular column of light. The effect wasn't as spectacular as hoped, however, since in the clear desert air the beam was generally invisible to passer-bys, except when a dust storm was in progress, when nobody wanted to be outside where they could enjoy the view. Of course, there are fountains that provide nighttime spectacles by shining colored lights up into the fountain jet, which provides plenty of scattering.
* Light is a transverse wave. This means that it is "polarized", with an angle of oscillation ranging 360 degrees around its direction of propagation. A "filter" can be designed to pass light of one polarization, but not another. For example, the lenses of "Polaroid" sunglasses contain long-chain molecules that are all oriented in a straight line in one direction. Light can pass through the lens if the polarization is in the same direction as that of the molecules, but not at other polarization angles. Reflections from horizontal surfaces tend to produce horizontally polarized light, and Polaroid sunglasses use vertical polarization filters to get rid of this glare.
It's a fun little game to take two sets of Polaroid sunglasses and look through the lenses of them in series at right angles to each other; the light is blocked out. (However, as described later, there is a very devious bit of fine print to this experiment.)
BACK_TO_TOP* Of course, we sense light through our eyes. The human eye has two classes of sensors, named "rods" and "cones". Rods provide perception of large patterns in the field of view, while cones provide detail and color perception. There are three types of cones, with each sensitive to a different region of the visible-light spectrum:
Incidentally, this arrangement was discovered by Thomas Young, whose formal training was as a medical doctor. Young was one of the truly brilliant figures in the history of science, with his accomplishments even including the deciphering of Egyptian hieroglyphics. Unfortunately, he was the sort of genius who could not communicate with others on their own level, and he was a notoriously tiresome lecturer.
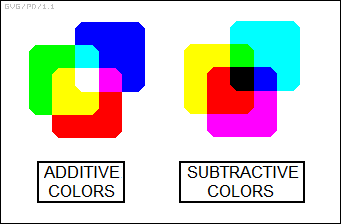
The perception of colors depends on the ratio of stimulation of these three types of cones by light, or in other words on the ratio of the red, green, and blue (RGB) components. These three "primary colors" add as follows:
_______________________________
red + green + blue -> white
red + green -> yellow
red + blue -> magenta
green + blue -> cyan
_______________________________
Different shades are produced by varying the level of each of the red, green, and blue components. Such an "additive" scheme is used to generate shades of color on a color TV or a computer display. The display consists of a grid of triplicate red-green-blue picture elements or "pixels"; the triplicate RGB components in each pixel are activated to different levels to give a composite resulting color. Typically, each of the RGB components can be set to one of 256 different shades from dark to light, resulting in 256 x 256 x 256 = 16,777,216 possible colors.
Also incidentally, there was an early color TV scheme that actually generated the red, green, and blue images sequentially -- one after the other, scanning them out rapidly enough so that it came out as a full-color image. The three images were all generated in black and white on an internal screen and then projected to a viewing screen through a rotating RGB color wheel. The scheme was simple and effective, but such a TV did not work with the black and white TV broadcast signals that were then the standard, and it was not successful. Astronauts on the later Apollo Moon landings did carry a sequential-color TV camera, since its simplicity allowed it to be made much smaller and lighter than the conventional parallel color TV cameras then in use. This sequential camera was designed specially for the Apollo project, and the sequential images were converted to standard color signals after being radioed back to Earth.
* Somewhat confusingly, printed color images use a complementary "subtractive" color scheme, based on yellow, magenta, and cyan dyes.
___________________________________
yellow + magenta + cyan -> black
magenta + cyan -> blue
yellow + cyan -> green
yellow + magenta -> red
___________________________________
The subtractive scheme is based on the fact that pigments are passive reflectors of ambient light, which is generally close to white light. Magenta pigment, for example, reflects blue and red light but absorbs green. Since yellow pigment reflects red and green light but absorbs blue, then a mix of yellow and magenta absorbs both blue and red light, while reflecting green. Mix yellow, magenta, and cyan paint together, and it absorbs all colors, leaving only black.
Perceived colors are also dependent on brightness, since rods tend to dominate over cones as light levels drop. The eye is very sensitive to colors, able to distinguish hundreds of thousands of different colors. However, the eye's ability to automatically compensate for changes in brightness makes it very poor at discriminating light levels, with tests showing the number of levels it can discriminate as no more than about 30.
The eye's structure and the layers of nerve cells that support that structure are optimized for certain tasks, such as determining the edges of objects and their motion. The eye, interestingly, is not very sensitive to changes in shade across a scene. A large square that varies gradually in shade from side to side will appear as one color.
* It has to be emphasized that there is nothing fundamental, at least from the point of view of pure physics, about the colors red-green-blue (or yellow-magenta-cyan). They are just arbitrary bands of light. The only reason they are special in any way is because the trial-and-error motions of evolution just happened to yield cones that were sensitive to these three wavelength bands. Different classes of other animals with color vision have different color reception schemes; instead of three different types of color receptors, most mammals only have two, while birds often have four and can see into the ultraviolet. Nocturnal animals -- which are active at night and sleep during the day -- generally don't have color vision at all.
BACK_TO_TOP