
* The increasingly detailed understanding of the physicists about the nature of the atom gradually led to the ability to manipulate the nuclear structure of atoms. At first such manipulations were strictly lab experiments with little practical application, but it was soon realized that a process for breaking the nuclei of heavy atoms, performing "fission", would not only release energy but could also produce a runaway "chain reaction". The result was the atomic bomb, as well as controlled nuclear power.
Further work on nuclear physics led to the understanding of "fusion" processes, in which tritium was fused together to produce helium. The end product of this effort was the "hydrogen bomb", which was far more powerful than the atomic bomb. More peacefully, the race to build nuclear weapons also led to a scientific understanding of how the atomic elements came to be in the first place.
* Radioactivity directly implied the breakdown of atomic elements. This led to the obvious question of whether atomic nuclei could be built up as well.
In 1919, following up a 1915 set of experiments conducted by his student Ernst Marsden, Rutherford publicly announced the results of an exacting set of experiments in radioactive phenomena. He placed a bit of radioactive material at one end of a sealed, evacuated cylinder; the cylinder was coated with zinc sulfide at the other end. The radioactive material produced alpha particles that traveled down the tube, striking the zinc sulfide and emitting "scintillations" of light. The zinc sulfide acted as a primitive type of "scintillation detector".
If the cylinder was filled with hydrogen gas, the scintillations were particularly bright. That was due to simple considerations of momentum: if an alpha particle struck a hydrogen nucleus -- a single proton, effectively four times lighter than an alpha particle -- then the collision would transfer momentum to the proton, giving it a velocity up to four times greater than the alpha particle. If the cylinder was filled with oxygen or carbon dioxide, both of which were substantially heavier than alpha particles, the scintillations would be damped.
However, if the cylinder was filled with nitrogen, the same bright scintillations occurred. Rutherford guessed that the alpha particles were knocking protons out of nitrogen nuclei. The collisions led to the absorption of an alpha particle into the atomic nucleus and the emission of a proton, resulting in the synthesis of an oxygen-17 atom (atomic number 8) from a nitrogen-14 atom (atomic number 7). That was confirmed in 1925, when the British physicist Patrick Maynard Stuart Blackett (1897:1974) decided to follow up on Rutherford's experiment to see what was going on in more detail. He used a cloud chamber -- a box with a glass lid containing humid air, with charged particles leaving visible tracks through it -- to observe interactions between alpha particles and nitrogen, taking 20,000 pictures, observing 400,000 alpha particle tracks, and discovering the synthesis of a grand total of eight oxygen atoms from collisions between alpha particles and nitrogen atoms. He would win the Nobel Prize in 1948 for this work.
* Rutherford was the first to observe transmutation of the elements through radioactive decay, and also the first to perform transmutation of the elements through "nucleosynthesis". That gave him a double justification for being labeled an alchemist. Many others wanted to get in on the act in his wake. Nucleosynthesis was an intriguing notion in itself; as the Latvian-American chemist Aristid V. Grosse (1905:1985) suggested in 1932, it also presented the possibility of creating isotopes that weren't actually found in nature.
In 1934, the Joliot-Curies were following up the experiments that had led to the discovery of the neutron, bombarding an aluminum foil target with alpha particles generated from a radioactive source, and pulling the source back from the target to investigate the effect of lowering the energy of the particles on neutron emission from the target. The experiment was fairly cut-and-dried, but it yielded a surprise: if the alpha particle source was removed, the target kept on emitting radiation, with the level falling off over a few minutes, as if some short-lived radioactive isotope had been produced by the bombardment.
After double-checking their work, the Joliot-Curies found out that there was indeed a radioactive material in the aluminum foil, with a half-life of 2.6 minutes. Their analysis suggested that the alpha particles had converted aluminum-27 (atomic number 13) into phosphorus-30 (atomic number 15), ejecting a neutron in the process. In search of proof of this notion, a helpful chemist showed them how to dissolve the irradiated aluminum in acid, with the phosphorus combining with hydrogen and then drawn off as a gas. The gas was radioactive while the aluminum in acid was not, and the gas turned out to contain phosphorus-30.
Phosphorus-30 was unstable, decaying with the half-life of 2.6 minutes as observed by the Joliot-Curies. Due to its short half-life, and also due to the fact that phosphorus-30 was not a link in any known radioactive decay chain, phosphorus-30 was not found in nature since any that was created promptly disappeared. The experiment was very significant, making up for the couple's embarrassment at missing the neutron, and they were awarded the Nobel Prize in 1935 for the discovery.
* Other short-lived radioactive isotopes not found in nature were then synthesized. There was a problem with using alpha particles for nucleosynthesis, however: they were positively-charged, and so were repelled by the positively-charged nucleus of the target atoms. As atomic number increased, the number of positive charges in the nucleus increased. Alpha particle penetration became increasingly difficult, until above a certain atomic number it wouldn't take place at all.
Following the discovery of the neutron, Enrico Fermi wondered if it might be a better tool for nucleosynthesis, since it was neutral and could in principle penetrate any nucleus, no matter how large. The fact that the neutron was uncharged also made producing a stream of them a bit tricky, but Fermi found that he could bombard a block of paraffin with protons, with collisions generating the desired stream of neutrons.
Fermi, working with his colleagues in Corbino's lab, started by bombarding hydrogen with neutrons and gradually worked his way up the periodic table. He got nowhere until 21 March 1934, when he bombarded fluorine with neutrons and ended up with a radioactive fluorine isotope. He soon began to synthesize other radioactive isotopes. Initially, Fermi thought high-energy fast neutrons would be best for his studies, but then he found out that he got better results if he placed the neutron source on a wooden table instead of a marble one. Fermi had a hunch that the wooden table was slowing down the neutrons. He tried using water or paraffin to slow down the neutron stream and found, somewhat to his surprise, that slow neutrons had a greater probability of interacting with nuclei. It was pointed out later that in this particular case, being Italian had paid benefits all by itself: marble tables are relatively common in Italy, fairly rare elsewhere, and in another country the connection might not have been noticed for some time.
Of course, since neutrons could penetrate heavy nuclei while alpha particles couldn't, Fermi and his people went on to bombard uranium (atomic number 92) with neutrons. He did seem to get an element with atomic number 93, but the results of the experiment were very confusing, and it would take a while to straighten them out.
* The Italian physicist Emilio Segre (1905:1989), a colleague of Fermi's in Corbino's lab, decided to investigate another angle on the matter. One of the mysteries of science at the time was that there were a number of vacancies in the periodic table of the elements, at atomic numbers of 43, 61, 85, and 87, where no element could actually be found in nature. In 1937, Segre went to the United States where he bombarded molybdenum (atomic number 42) with neutrons, and managed to synthesize element 43. He wasn't quite sure it was a proper element, but after World War II the scientific community came to a consensus that it was, and so Segre gave his element the name "technetium". The reason it wasn't found in nature is obvious in hindsight: it had no stable isotopes. It did have three radioactive isotopes, the longest-lived being technetium-97, with a half-life of 2,600,000 years. That's a long time in human terms, but not so long by cosmic standards, and any technetium formed by natural processes gradually disappears below the noise level.
The other three vacancies in the periodic table had also been plugged by that time. Element 87 was discovered in 1939, to be named "francium" in honor of the nation in was discovered; element 85 was discovered the next year, 1940, to be named "astatine"; and element 61 was discovered in 1947, to be named "promethium". To no surprise, none of these elements had stable isotopes either, and even their most stable isotopes didn't have very long half-lives. Francium-223 has a half-life of 21.8 minutes, astatine-210 has a half-life of 8.1 hours, and promethium-145 has a half-life of 17.7 years,
* That filled in the vacancies in the periodic table, but what about the unfilled entries above the end of the table at uranium, element 92? As mentioned, Fermi had thought he had created element 93 in 1934 but hadn't been sure enough to make the claim that he had. In 1940, two American physicists, Edwin Mattison McMillan (1907:1991) and Philip Hauge Abelson (1913:2004), repeated Fermi's experiment, bombarding uranium with neutrons, and produced element 93. Since the planet Uranus was followed by the planet Neptune, they logically named the next element beyond uranium "neptunium".
Neptunium has no stable isotopes -- no element above uranium does -- though its longest-lived isotope, neptunium-237, has a half-life of 2,140,000 years. The half-life of neptunium-237 is still too short for it to persist in nature. Incidentally, neptunium also parents a decay series similar to those produced by uranium and thorium.
The discovery of neptunium led directly to the next element. McMillan and another American physicist, Glenn T. Seaborg (1912:1999), found that neptunium could perform beta decay, releasing an electron to convert a neutron into a proton and creating element 94. Using the same naming convention of following the order of the planets as they were known at the time, they named the element "plutonium". Its longest-lived isotope is plutonium-244, with a half-life of 82,000,000 years.
Seaborg had to keep his discovery secret for the time being; the British had discreetly protested the publication of the discovery of neptunium, since it clearly gave important clues to Nazi scientists, and atomic scientists in the US had agreed to perform self-censorship on their discoveries. This would prove wise, since plutonium would have, to put it mildly, military applications.
* Since that time, the list of transuranium elements has been brought up to element 118. The following table lists all transuranium elements known as last notice, along with their dates of discovery.
element name date __________________________________ 93 neptunium (Np) 1940 94 plutonium (Pu) 1940 95 americium (Am) 1944 96 curium (Cu) 1944 97 berkelium (Bk) 1949 98 californium (Cf) 1950 99 einsteinium (Es) 1952 100 fermium (Fm) 1955 101 mendelevium (Md) 1955 102 nobelium (No) 1958 103 lawrencium (Lr) 1961 104 rutherfordium (Rf) 1969 105 dubnium (Db) 1970 106 seaborgium (Sb) 1974 107 bohrium (Bh) 1976 108 hassium (Hs) 1984 109 meitnerium (Mt) 1982 110 darmstadium (Ds) 1994 111 roentgenium (Rg) 1994 112 copernicium (Cn) 1996 113 nihonium (Nh) 2004 114 flerovium (Fl) 1998 115 moscovium (Mc) 2004 116 livermorium (Lv) 2000 117 tennessine (Ts) 2010 118 ogannesson (Og) 2002 __________________________________
One of the interesting speculations about nuclear physics is that there may be even heavier elements that are relatively stable. Nuclear researchers long ago noticed that nuclei with certain "magic numbers" of protons or neutrons are relatively stable. Such magic numbers correspond to filled nuclear energy shells, analogous to the way that elements with filled electron energy shells are inert.
The numbers 2, 8, 20, 28, 50, and 82 are known to be "magic" for both protons and neutrons, and nuclei that have magic numbers for both protons and neutrons are unusually stable, or "doubly magic". The highest known magic number for neutrons is 126. The normal lead atom is doubly magic, with 82 protons and 126 neutrons, making it more stable than any known element higher in the periodic table. The next higher magic proton number may be 114, 120, or 126; the next higher magic neutron number is agreed to be 184. That implies that if nuclear scientists could get over the barriers that block construction of heavier nuclei, they might obtain atoms that are surprisingly stable. For now, however, they remain the stuff of science fiction.
BACK_TO_TOP* One of the interesting things that Francis Aston discovered as he was sorting out the isotopes of atoms and their ratios was that, even after determining the mass of a particular isotope, it was slightly less than would be expected by adding up the masses of the appropriate number of individual protons and neutrons needed to make up that nucleus. This was known as the "mass defect", and Aston quickly realized that it was Einstein's E = MC^2 relationship in operation. In binding together, some of the mass of the nucleons was converted into energy, released, and lost. Not surprisingly, this energy was referred to as the "binding energy". Physicists defined a "packing fraction" to give the relative mass of the nucleus relative to its "expected" mass:
10,000 * ( ( expected_mass - actual_mass ) / expected_mass )
The factor of 10,000 is included because the mass defect is usually very small. For example, carbon-12, with six protons and six neutrons, should have a mass of 12.096, but in reality, it has a mass of 12. This gives a packing fraction of: 10,000 * ( 0.096 / 12.096 ) = 80. If packing fractions are calculated for the elements, it turns out that the value increases up to iron-56, with a packing fraction of 94, and then decreases again consistently for heavier elements. Uranium-238 has a packing fraction of only 79.4. In principle, to break an atom up into its constituent individual protons and neutrons requires addition of exactly enough energy to match, through E = MC^2, the mass defect represented by the packing fraction.
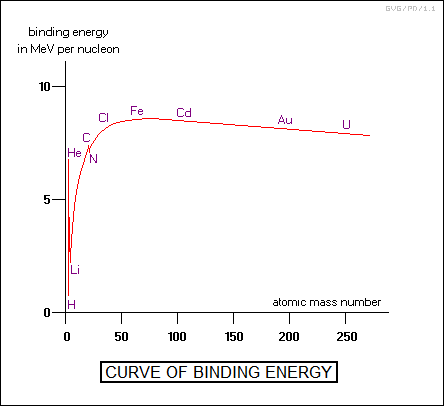
The nucleosynthesis of "light" elements, in this context meaning lighter than iron-56, will in general release more energy than put into the process, while nucleosynthesis of "heavy" elements will in general require a net input of energy. Nature does have a tendency towards the most favorable energy state, the most obvious example being the fact that rocks roll to the bottom of ridges, and that leads to the question of why the entire Universe isn't composed of iron-56. After all, the radioactive decay series of heavy elements moves towards lower atomic numbers, towards if not ending at iron-56. However, stable isotopes generally are just that, stable, and it takes a substantial threshold of energy to change them into different isotopes. Rocks may roll to the bottom of a ridge, but if there is a series of ridges, each starting from a low altitude on one side and leading to a valley at a higher altitude to the next ridge, a rock is not going to roll by itself from the top of the highest ridge to the bottom of the lowest, even though it will obtain the lowest energy state by doing so.
* In 1934 Enrico Fermi had bombarded uranium with neutrons and obtained very odd results. He did suspect he had synthesized element 93, but he couldn't be sure because the bombardment also created a range of products of lower atomic weights than uranium. Despite the uncertainties in his experiment, he received the Nobel Prize for it in 1938. Another physicist named Ida Tacke (1896:1978) who had been tinkering with nucleosynthesis suggested that since the uranium existed at the limit of the struggle between the EM and strong forces, the atom was likely to be easily fragmented and so neutron bombardment had simply shattered it into pieces. The suggestion was a bit too far ahead of its time and wasn't taken very seriously. As often happens in such circumstances, it would take practical research instead of informed speculation to change mindsets.
In 1937, two chemists, a German named Otto Hahn (1879:1968) and an Austrian named Lise Meitner (1878:1968), then collaborating in Germany, decided to methodically follow up Fermi's 1934 experiment. Initially, Hahn simply thought that neutron bombardment had knocked two alpha particles out of the uranium nucleus, reducing from uranium, element 92, to radium, element 88.
However, sifting the trace quantities of radium out of bombarded uranium was a tricky task. The approach they took was based on the fact that barium, element 56, sat directly above radium, element 88, in the next row of the periodic table, and so the two elements had similar chemical properties. Hahn and Meitner decided to dissolve their bombarded uranium samples in acid, add a stable barium isotope to the solution, and then separate out the barium. The radium would separate out along with the barium; any radioactivity in the separate could only be due to traces of radium. Since the radioactive intensity of radium was known, the proportion of radium could be determined from the level of radioactivity.
In 1938, Adolf Hitler annexed Austria into the Greater German Reich and Lise Meitner automatically became a German citizen. Since she was Jewish, that was far from welcome news. The Netherlands agreed to admit her on her old, now obsolete, Austrian passport, so she fled Germany, suffering through a moment of terror when German soldiers inspected her passport, to finally end up in Sweden.
Hahn joined forces with another German chemist, Fritz Strassman (1902:1980), to continue the hunt for radium in bombarded uranium. They did manage to extract radioactive barium from a bombarded uranium sample, but attempts to actually extract radium from the separate failed. Hahn finally decided that the separate included a radioactive isotope of barium. It strongly suggested that neutron bombardment had shattered the uranium atoms, or in other words performed "atomic fission". Hahn was reluctant to come out and make this claim, but he still got the Nobel Prize for this work in 1944.
Lise Meitner was keeping up with the effort, and came to the conclusion that fission had occurred. She collaborated with her nephew, the physicist Otto Frisch (1904:1979), who was then working in Niels Bohr's lab in Copenhagen, to write a letter for the British science journal NATURE, and submitted it in early 1939. Frisch told his boss Bohr about the letter, and before January 1939 was out, Bohr had attended a physics conference in Washington DC, where he spread the news: atomic fission had been performed.
BACK_TO_TOP* The discovery of nuclear fission had immediate and dramatic consequences. Leo Szilard (1898:1964) was a Jewish Hungarian physicist who had left Berlin for Britain in 1933, after the Nazis had passed a decree effectively throwing all academics of Jewish extraction out of their positions. He went on to the US in 1938, signing on with Columbia University in New York City and becoming an American citizen in 1943.
In 1933, Ernest Rutherford had described the idea of atomic power as "moonshine". He knew perfectly well that there were enormous energies locked up in the atom, but he couldn't see that there was any way to release those energies except through radioactive decay, which was a stingy process at best. It was an unfortunate comment. Rutherford was a man of enormous accomplishments; his deceptively simple experiments were implemented with a level of care and craftsmanship compared by some physicists to that needed to build a Stradivarius violin, and his lab generated almost a dozen Nobel Prize winners. However, the times were beginning to pass him by. Rutherford would die in 1937, of an infection following relatively minor surgery, not living long enough to find out how spectacularly off target he had been in his assessment of the potential of atomic energy.
In 1934, annoyed at a newspaper article that cited Rutherford's "moonshine" remark, while walking the streets of London, Szilard had realized that nuclear fission might be the key to building a super-powerful "atomic bomb" through a "chain reaction": the fission of one atom by neutrons releasing more neutrons to perform fission on other atoms, leading to a cascade that released a vast amount of energy. Chain reactions were not particularly part of physics -- but they were known in chemistry. Szilard contacted the British chemist Chaim Weizmann (1874:1952), a prominent Zionist and later first president of Israel, to see if it could be done, but the experiments went nowhere.
Szilard had originally focused on producing a chain reaction in the relatively light element beryllium, which seemed a promising line of investigation at the time, but didn't turn out to be a very good candidate. Lise Meitner's paper on nuclear fission in uranium opened the door. Weizmann's experiments had used energetic fast neutrons to perform fission, but Fermi's work established that slow neutrons worked better, and if the fission released slow neutrons, a chain reaction might well be possible. Further research indicated that was the case.
Szilard managed to convince his colleagues to keep quiet about research into fission processes, and in 1939 he contacted two other Hungarian physicists in the US -- Eugene Paul Wigner (1902:1995) and Edward Teller (1908:2003) -- to collaborate on the development of the atomic bomb: it might be a terrible weapon, but it would be even more terrible if Hitler got it first. Szilard was a friend of Albert Einstein's, the two having collaborated on the invention of a commercially unsuccessful refrigerator of all things, and Szilard knew that Einstein's prestige would help get a warning on the matter to the highest levels. The three Hungarians visited Einstein and persuaded him to write a letter to US President Franklin Delano Roosevelt to propose that the United States begin a high-priority program to build an atomic bomb.
The letter, dated 2 August 1939, was personally delivered by economist Alexander Sachs, who had close access to the president. In response, the US government set up a committee and provided a grant of the princely sum of $6,000 USD for research. The grant would be delayed until early the next year, 1940. By that time, World War II was in full swing, and many European physicists who had fled the Nazis were becoming concerned about the atomic bomb and the idle way the US government was investigating it. Some prominent physicists had remained in Germany, most prominently Werner Heisenberg, and they might well be working on the Bomb for Hitler.
* Enrico Fermi was also at Columbia. In 1938, he was awarded the Nobel Prize for his work on transmutation of the elements. Antisemitism was on the rise in Italy, and his wife was Jewish; when he went to Stockholm to be awarded his Nobel Prize, he took his family with him, and they didn't go back. He and Szilard decided to collaborate on building an "atomic pile" using uranium oxide in which controlled nuclear chain reactions could be performed for research purposes.
To have a sustained chain reaction, the average number of neutrons ejected from the fission of a uranium atom to cause the fission of another uranium atom had to be greater than 1. In more formal terms, the "reproduction factor k" had to be at least one. Studies had shown that on average, uranium releases 2.5 neutrons per fission event, which put the maximum value of k as 2.5. Although that was much more than needed for a self-sustaining chain reaction, neutrons were invariably lost during the fission process, and so obtaining a chain reaction was not simple. To complicate matters considerably, although it might be hard to start a chain reaction, nuclear processes occur very quickly, and even a reproduction factor slightly greater than one would cause a chain reaction increasing exponentially, cascading out of control in a hurry.
Fortunately, the researchers discovered that a small fraction, less than a percent, of the neutrons emitted by the fission process were "delayed" neutrons. These delayed neutrons were emitted by the fission fragments after a certain time delay, instead of being emitted by the fission action itself. The time delay involved was surprisingly long, on the order of ten seconds. This means that if the value of k was between 1 and 1.01, the delayed neutrons decided the balance of the chain reaction, and the reaction was slow enough to be controlled.
The pile was built out of blocks of graphite, which would act as a "moderator", slowing down neutrons to allow them to more easily produce fission reactions, while not absorbing the neutrons and ensuring that the neutron flux was not damped. The graphite had to be very pure, since even small traces of neutron-absorbing materials in the graphite could damp the chain reaction. Prototype piles were assembled at Columbia to test out concepts,
Funds remained thin for much of the year, but by October 1941 the US government had increased support. After the Imperial Japanese Navy bombed Pearl Harbor on 7 December 1941, throwing America into World War II, the project began its rise to the top of the priority list. Arthur Holly Compton, then at the University of Chicago, was put in charge of the project, with his organization known as the "Metallurgical Laboratory" as a cover. Szilard and Fermi went to Chicago in the spring of 1942 to work in the lab.
The Metallurgical Lab built a further series of test piles, with work going on around the clock and the k factor steadily increasing. Finally, late in 1942, the group assembled a pile that would be able to perform a controlled chain reaction. On 2 December 1942, workers carefully pulled out cadmium control rods that had been inserted into the pile to dampen a chain reaction, That afternoon, Fermi announced: "The reaction is self-sustaining." The pile ran for 11 minutes and was shut down. They were now "moonshiners".
BACK_TO_TOP* Achieving a controlled chain reaction was a tricky business. The most common isotope of uranium, uranium-238, is not well suited for it. Fission will occur spontaneously in nature, though it is relatively rare compared to normal radioactive decay processes such as alpha decay because fission has a fairly high "activation energy". Uranium-238 will undergo spontaneous fission once for every 220 times it emits an alpha particle. Uranium-238 also requires fast neutrons for fission, not slow neutrons, and only releases slow neutrons, meaning it can't support a chain reaction.
Niels Bohr suggested that the less common uranium-235 isotope would be much better suited to generating a chain reaction. It is much less stable than uranium-235, with a half-life only a sixth as long, and can be split by slow neutrons. Since uranium-235 is only about 1% of a natural sample of uranium and has very similar properties to other uranium isotopes, separating it was a major challenge. Separation of isotopes still remains the major challenge to nations intent on developing nuclear weapons.
Uranium-238 wasn't inherently useless, however. It could be bombarded with neutrons to convert it into neptunium-239 and then into plutonium-239, which could support a chain reaction and had a long enough half-life -- 24,000 years -- to allow it to be stockpiled. Similarly, although thorium-233 is not in itself useful as a nuclear fuel, it could be converted by neutron bombardment into uranium-233, which will also support a chain reaction, and has a half-life of 160,000 years.
Following the demonstration of a controlled chain reaction, the US moved into high gear to build an atomic bomb under what became informally known as the "Manhattan Project", with research and development centered at Los Alamos in New Mexico. The project was under the technical direction of Robert Oppenheimer (1904:1967) of Caltech and the University of California at Berkeley. Top minds from the US and Europe worked on the project, though Einstein's contribution to the effort was basically restricted to writing the letter to Franklin Roosevelt. Large-scale separation plants were built, and the first large-scale nuclear reactors were put into operation at Hanford, Washington state, in 1944, using the fission of U235 to bombard U238 with neutrons and "breed" P239.
Despite the huge industrial infrastructure needed to build the Bomb, it was an indication of the magnitude of American resources that the program moved forward quickly, in fact ahead of schedule. The design team had fears at one time that setting off a nuclear weapon might ignite the Earth's atmosphere, but theoretical studies showed it was well outside the bounds of the possible. The first atomic bomb, the "Trinity" test, was detonated on 16 July 1945 at Alamagordo, New Mexico; local newspapers were fed news that an ammunition dump had exploded to explain the resulting loud bang and mushroom cloud. The researchers had bet on the yield, with the yield coming in on the high side of the estimates. Much to the annoyance of the authorities, Enrico Fermi had also flippantly tried to set up a betting pool on the probability that Trinity still might set the atmosphere alight.
Operational weapons were not far behind. On 6 August 1945, less than a month later, a uranium-235 device known as "Little Boy" was dropped on the Japanese city of Hiroshima. On 9 August 1945, a more powerful plutonium-239 device known as "Fat Man" was dropped on the city of Nagasaki. Fat Man, incidentally, was an operational version of Trinity; astoundingly, Little Boy was not test-fired before being dropped on Hiroshima, such was the confidence that it would work, the only question being yield. Japan surrendered on 14 August 1945. These were the only two nuclear weapons to ever be used in combat -- so far.
As revealed by Allied military intelligence after the war, much to the surprise of the physicists who had worked on the Manhattan Project, the Nazi Bomb project lacked direction and resources, and ended up being focused on reactor development, a Bomb being regarded as too ambitious. Heisenberg had in fact been a major player in the project, but the most he really accomplished was to taint his reputation with the professional physics community for the rest of his life. The Japanese did some research on nuclear weapons as well, but the effort was even more threadbare than German work on the Bomb.
However, the Soviet Union, aided by well-placed spies in the Manhattan Project, was moving full steam ahead, and to the shock of Americans the USSR detonated the first Red Bomb in 1949. In the 1950s, the US and the USSR engaged in a massive nuclear arms buildup, while atomic reactors were put to use to provide power for both military and civilian uses. Nuclear power is discussed in more detail later.
BACK_TO_TOP* The topics of nucleosynthesis and atomic power lead almost inevitably to the question of how the elements in the Universe were synthesized in the first place.
The answer to this question related to the energetic processes that fuel stars. In the days before physicists had any understanding of nuclear processes, the general notion was that the Sun and other stars were simply balls of fire. An analysis of the combustion of a ball of burning material the size of the Sun gave an age to the Universe that was gradually seen as unconvincingly short.
The assumption that the Sun and the stars were glowing from the energy produced by gravitational compression in their formation seemed to be more workable. The German physicist Hermann von Helmholtz (1821:1894) and the American astronomer Simon Newcomb (1835:1909) independently calculating the amount of time it would take for the Sun to condense down to it current diameter and brightness from the nebula of gas and dust from which it was born. They came up with a value of 100 million years. Unfortunately, after radioactive dating was invented, studies performed with the technique suggested that still wasn't anywhere near long enough.
However, the discovery of radioactive decay revealed the energy locked up inside the atom, providing a power source that could keep stars glowing for vast lengths of time. Nobody understood the specifics of how the atoms powered stars at first, but in 1926, the British physicist Sir Arthur Stanley Eddington (1882:1944) published a book titled THE INTERNAL CONSTITUTION OF STARS, which essentially founded modern astrophysics. He suggested that the high temperatures and pressures at the core of the Sun and other stars could force hydrogen nuclei to fuse into helium nuclei, and such "fusion" processes would be able to provide enough energy to keep the Sun and other stars burning for a long, long time.
Eddington did not have all the precise details of such fusion reactions, but other physicists, initially George Gamow, then the German astrophysicist Carl von Weisaecker and the German-American astrophysicist Hans Bethe (1906:2005), filled in the blanks. Bethe, not so incidentally, was a major player in the Manhattan Project. Once physicists understood the details of stellar hydrogen fusion, they understood that it would be possible to use a fission bomb to initiate hydrogen fusion, resulting in the vastly powerful "fusion weapons" developed in the 1950s. Bethe was a contributor to this design, though the core ideas were developed by Edward Teller and the Polish-American mathematician Stanislaus Ulam (1909:1984).
The physics community in both West and East remained preoccupied with weapons design from the early 1940s well into the 1950s, but then, the basic designs of weapons as powerful as anyone would want in their most lunatic dreams having been established, work began to drift back towards more theoretical subjects. Further work in astrophysics detailed the life cycles of stars and showed how heavier elements could also be synthesized by fusion and other processes.
There was the other question of where the material to build the stars had come from to begin with. In the modern era, up through the 1950s, there were two competing theories:
Cosmology is a subject at least partly outside the domain of quantum physics and so discussing it in any detail here would be inappropriate. It should be enough to say that in the 1960s the balance had tipped decisively towards the Big Bang theory, mostly because observations determined the entire sky had a faint microwave emission that was clearly the cooled remnant of the black-body energy of the "primordial explosion".
In outline, as understood today, the Big Bang occurred 13.7 billion years ago, producing a Universe composed of about three quarters hydrogen, one quarter helium and wisps of a few slightly heavier elements. The hydrogen formed into stars, performing fusion reactions, initially fusing hydrogen into helium. Larger stars could perform successive fusion reactions, creating heavier elements; the largest stars would synthesize the elements up to iron; and then, out of fuel, collapse in on itself and explode as a "supernova".
There was long a belief that supernovas would breed elements above iron, but it wasn't clearly understood how. More recent thinking suggests the heavier elements were synthesized by the collisions of pairs of superdense neutron stars, but there's still dispute over that, too. There may have been a number of alternative paths; research continues.
BACK_TO_TOP