
* The notion that the chemical elements were based on units known as "atoms" emerged through the 19th century. In the early 20th century, it became increasingly obvious that atoms, which had been regarded as fundamental, were made up of smaller particles. Investigation of the structure of the atom helped lead to a revolution in physics.
* The concept of the basic particle of matter, the atom, goes back at least to ancient Greece and classical India. These early notions of atoms, however, were philosophical, simply an assertion without experimental validation that matter was not indefinitely divisible.
It was the chemical revolution of the late 18th and early 19th century that brought atoms into the realm of science. That was due primarily to the work of the English chemist John Dalton (1766:1844), who published his atomic concepts in his book A NEW SYSTEM OF CHEMICAL PHILOSOPHY, published in 1808. Dalton proposed that:
Trying to determine the ratios of elements in chemical compounds proved tricky, but Dalton was on the right track, with the first workable table of the elements published by the Russian chemist Dmitriy Mendeleyev (1834:1907) in 1869. Mendelyev's "periodic table" was incomplete, but by the end of the century, it had generally been populated, with the properties of the elements in the table generally understood.
By that time, however, it was becoming apparent that atoms were not indivisible. In 1897, the English physicist J.J. Thomson (1856:1940) determined that the electrically-charged beam in a cathode-ray tube was made up of negatively-charged particles. He was able to determine the ratio of charge to mass of these particles; in 1913, the American experimental physicist Robert Millikan (1868:1953) would publish research revealing the charge of the electron, and so its mass. It turned out to be 1,800 times lighter than a hydrogen atom. They became known as "electrons", and were determined to be the usual component of electrical currents.
In any case, it was also realized that removal of electrons from atoms left behind positively-charged ions. Thomson suggested that electrons were distributed throughout an atom, which became known as the "plum pudding" model of an atom.
In 1896, the French physicist Henri Becquerel (1852:1908) discovered that uranium salts emitted an invisible radiation. Becquerel had discovered radioactivity; further research showed that radioactivity resulted in transmutation of the elements. Atoms that emitted radiation were transformed into lighter elements, as if they had lost part of their fundamental structure.
Two French chemists, the husband and wife team of Pierre Curie (1859:1906) and Polish-born Marie Curie (1867:1934), were able to demonstrate that radioactivity was a property of several types of atoms. The Curies determined that the "decay" of radium produced heating effects that cumulatively provided far more energy than could be provided by burning an equivalent mass of, say, coal. Becquerel and the Curies would share the 1903 Nobel Prize in physics for the discovery of radiation.
In 1909, the German physicist Hans Geiger (1882:1945) and the English physicist Ernest Marsden (1889:1970), working at the University of Manchester in the UK under the direction of the New Zealander physicist Ernest Rutherford (1871:1937), bombarded a metal foil with alpha particles -- in effect, ionized helium -- to observe how they scattered. They expected that the alpha particles would to pass through with little deflection, since Thomson's plum pudding model suggested the atom was a diffuse structure.
However, Geiger and Marsden found alpha particles being deflected backwards, much to their astonishment. Rutherford concluded that there was a dense central mass, a nucleus, in an atom. In the Rutherford atom, negatively-charged electrons orbited around a central nucleus -- envisioned early on as like planets around the Sun, though that would prove an entirely misleading idea. In any case, it was soon realized that the nucleus of an atom contained one or more positive charges. These positive charges became known as "protons", which were effectively the same as ionized hydrogen atoms.
The problem with the Rutherford model was that electrons were attracted to the positively-charged nucleus -- so why didn't they just fall into the nucleus? In 1913, the Danish physicist Niels Bohr (1885:1962) proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes, quanta, of energy corresponding to absorption or radiation of a photon.
Bohr's model was strictly ad-hoc; it did not explain why there were sets of fixed orbits in an atom. In 1924, the French physicist Louis de Broglie (1892:1987) proposed that all particles had a wavelike aspect. That was why the electrons in an atom had only certain orbits; they correspondent to resonant states of the electrons.
In 1926, the Austrian physicist Erwin Schroedinger went on from this idea to develop a general equation in which electrons and other subatomic particles were described using a system of "wavefunctions". Using the Schroedinger equation, the electron orbitals were defined as statistical distributions, giving results that had little resemblance to a planetary system.
Roughly in parallel, the German physicist Werner Heisenberg (1901:1976) determined that, at the subatomic level, there was a hard limit of size, energy, and time, beneath which observations were literally impossible -- with Heisenberg's "uncertainty principle" constraining what could and could not be measured. The end result of such efforts, as systematized by Bohr, resulted in a new system of "quantum mechanics" that elaborated on the world described by the classical mechanics of traditional physics.
BACK_TO_TOP* The vision of the atom as consisting of a positively-charged nucleus containing protons orbited by electrons, was correct as far it went, but it left a puzzle. In 1911, the British physicist Charles Glover Barkla (1877:1944) had conducted experiments on the production of X-rays by bombarding anodes made of different metals with an electron beam. He discovered that different types of metals would produce X-rays with different energies, a discovery that won him the Nobel Prize in 1917.
Rutherford's student Henry Moseley (1885:1915) went further with the idea, observing that the energy of the X-rays increased as the elements grew heavier, but not at the same rate as the atomic mass. He suggested that the energy was actually proportional to the net positive charge of the nucleus, in terms of integer multiples of the charge of the proton. The greater the positive charge on the nucleus, the greater the deceleration of the electron, resulting in the emission of a more powerful X-ray photon.
The size of this positive nuclear charge became known as the "atomic number"; it was equivalent to the number of electrons in orbit around a neutral atom. Moseley suggested that the elements were ordered in the periodic table by their atomic number, which increased by a value of 1 for every successive element in the table. Now the periodic table started to make more sense.
Moseley's brilliant insight might have won him the Nobel Prize, but with the outbreak of the First World War, he went into the ranks of the British Army as a military engineer, to be killed in action during the disastrous landing at Gallipoli in Turkey. He remains a minor figure in the history of physics, but those who knew him and his work believed he had top potential. Robert Millikan wrote that the loss of Moseley all by itself made the conflict "one of the most hideous and irreparable crimes in history."
* To further complicate the picture of the atom, in 1913 Rutherford's student Frederick Soddy (1877:1956) had shown that there were different forms, or "isotopes", of the same sort of atoms. Soddy observed that there were some forms of uranium that were much more radioactive than others, even though all the forms were essentially identical in their chemical properties. He won the Nobel Prize in 1921 for this discovery. As it turned out, isotopes shared a common atomic number, but had different atomic weights. Uranium, for example, had an atomic number of 92, but had two primary isotopes, one with an atomic weight of 238 and another with an atomic weight of 235. These can be shorthanded as "U<238/92>" -- with the "238" read as a superscript and the "92" read as a subscript -- and "U<235/92>".
A relatively straightforward means of separating isotopes, known as a "mass spectrograph", was developed in 1919 by the British chemist Francis William Aston (1877:1945), one of J.J. Thomson's students. In the mass spectrograph, atoms are ionized, accelerated by an electric field, and thrown around a curve in a magnetic field to strike a target containing a set of detectors in a row. Their paths will vary slightly according to their masses, and so different isotopes will fall into different detectors. Aston won the Nobel Prize for chemistry in 1922; over the next two decades, he sorted out over two-thirds of the 282 naturally-occurring isotopes.
Some elements, such as sodium, aluminum, cobalt, and gold, have no isotopes normally observed in nature; others may have up to a half-dozen. The presence of isotopes had complicated the discovery that the nucleus of the atom was made up of elementary particles such as protons. In 1815, the British chemist William Prout (1785:1850) had suggested that all atoms were composites of hydrogen atoms, but his idea didn't pan out because estimates of the relative mass of elements in bulk showed they didn't have nice neat integer multiples of the mass of the hydrogen atom. The discovery of isotopes explained this: as Aston pointed out, elements obtained in purified bulk form included several isotopes, and the weights obtained were an average.
* By that time, the physicists were beginning to trace out the steps of various radioactive decay processes, observing the transformations of elements into their "decay products" as they released alpha particles (helium nuclei) and beta particles (electrons). Radioactive elements also emitted gamma radiation, but this only released energy and did not in itself lead to a transmutation of an element. Researchers were able to identify chains or "radioactive series" in these decay processes. The series could be complicated, with one radioactive element decaying to another radioactive element, and so on, until the decay chain finally came to rest in a stable element.
The researchers also began to measure the rates of decay for each step in the series. In keeping with the general spirit of quantum phenomena, there is no way to determine exactly how long it takes one atom of a radioactive element to decay into another, but for large numbers of such atoms the decay rate is very predictable. The radioactive decay rate is given traditionally in "half-lives", the amount of time it takes for half a given amount of radioactive material to decay. If such a material has a half-life of, say, two years, in two years there will be only half of it left; in four years, only a quarter; in eight years, only an eighth; and so on. Such an "exponential" decline implies that there will always be some "die-hards" among the atoms in the material that will stubbornly refuse to decay, though sooner or later, they will become effectively undetectable.
BACK_TO_TOP* By the late 1920s, there was a general belief that the nucleus of the atom consisted of one or more protons along with a lesser number of electrons embedded in that nucleus, resulting in a nucleus with a net positive charge. In radioactive decay, emission of an alpha particle would remove four protons and two embedded electrons from the nucleus, reducing the atom's atomic weight by four and atomic number by two; or emission of a beta particle would remove one embedded electron, leaving the atomic weight essentially the same but increasing the atomic number by one. This concept had the virtue of explaining how the nucleus held together. After all, if the nucleus was nothing but positively-charged protons, their mutual electrostatic repulsion would tear the nucleus apart, and the Universe would consist solely of hydrogen atoms. The embedded electrons, it seemed, "glued" the nucleus together.
That was sort of right, but not exactly on target. There were two problems with this model. The first was that, for the larger atoms, it was difficult to get the sums of the weights of the protons and electrons to come out to the observed value of the weight of the nucleus. The second, more important problem was that the spins of the particles didn't add up. For example, a nitrogen-14 nucleus has a spin of +/-1. Protons, like electrons, are fermions and have a half-integer spin of +/-(1/2). If the nitrogen-14 nucleus honestly consisted of 14 protons and 7 electrons, that meant 21 particles with half-integer spin, and there was absolutely no way to get them to add up to an integer spin.
The way out was to get rid of the 7 electrons and build the nucleus out of 7 protons and 7 particles that looked just like protons, but were electrically neutral. Instead of having discrete protons bound to discrete electrons in the nucleus, somehow the two might be merged together, forming a single particle in which the opposed electrical charges of the two balanced out. Rutherford had suggested this idea in 1920, and in 1921 the American chemist William Draper Harkins (1871:1951) named this particle the "neutron", meaning more or less "neutral proton". At the time, Harkins' neutron was a purely hypothetical beast, and most physicists didn't believe in it.
In 1930, a German physicist named Walter W.G.F. Bothe (1891:1957) and his colleague Hans Becker bombarded beryllium metal with alpha particles and managed to produce some sort of energetic radiation that was electrically neutral; for lack of a better idea, they suggested it was gamma radiation. In 1932, two French physicists, Frederick Joliot-Curie (1900:1958) and his wife Irene Joliot-Curie (1897:1956) -- Irene was the daughter of Pierre and Marie Curie -- published results of their experiments on this radiation. They found it ejected protons from a block of paraffin -- candle wax -- which was rich in hydrogen atoms as a source of protons. Gamma rays didn't seem adequate for the task, but the Joliot-Curies couldn't think of an alternative.
The British physicist James Chadwick (1891:1974) pursued the same line of investigation, but he was already hunting for the neutron and believed that the Joliot-Curies had, unknown to them, found the beast. Obviously, if this energetic radiation could eject protons, it had to be about as heavy as a proton; gamma rays simply didn't have the kick needed to do the job. Since the particles that made up the radiation were electrically neutral, that meant the particles were neutrons. Of course, he needed proof, and it had to be good proof lest he fall into the only-too-tempting trap of misinterpreting poor data just because he wanted to believe it. Chadwick duplicated the experiment performed by the Joliot-Curies, first obtaining protons from a block of paraffin, then substituting a wide range of other atoms for the paraffin. He got much the same results and published them immediately. The Joliot-Curies were deeply humiliated when they read Chadwick's report, to realize they had been overlooking the obvious.
Chadwick won the Nobel Prize in 1935 for his discovery. It took much longer to find the neutron than the proton because particle detector technologies developed up to then were only suited to tracking down charged particles; neutral particles like photons and neutrons don't leave ionization trails, and they can only be detected by direct interactions, in other words by slamming them into something. The neutron would prove to be very slightly heavier than the proton, by about 0.14%, with a mass of 1.675E-27 kilograms. Incidentally, protons and neutrons are collectively referred to as "nucleons".
* The discovery of the neutron made the concept of isotopes easier to understand: the nucleus of an atom of a particular element always contained the same number of protons, but it might contain different numbers of neutrons. For example, the nucleus of ordinary hydrogen contains only a single proton, but about one in every 7,000 hydrogen atoms has a nucleus with a proton and a neutron. This isotope is known as "deuterium" or "heavy hydrogen". Deuterium is a stable isotope; it does not undergo radioactive decay.
There is also an isotope of hydrogen with two neutrons, called "tritium", that is unstable, decaying with a half-life of 12.26 years into the helium-3 isotope, with two protons and one neutron. Helium-3 is stable but is about a million times less common than normal helium-4, which has a nucleus consisting of two protons and two neutrons. Of course, this is the same as the alpha particle.
All atoms of each particular element have, effectively by definition, the same number of protons, but the isotopes of an element have, again effectively by definition, a different number of neutrons. Uranium, near the other end of the periodic table, has 92 protons, with several isotopes: the most common, U<238/92>, has 146 neutrons, while the second most common, U<235/92>, has 143 neutrons.
* The discovery of the neutron unsurprisingly led to more questions. If the nucleus didn't contain electrons to glue it together, then how did the nucleus keep from flying apart? The neutron very likely had something to do with it, but what? There were also the related questions of why elements became more unstable as their atomic number increased, and why the ratio of neutrons to protons generally increased with atomic number as well.
In 1932 Heisenberg suggested that the nucleus was held together by a "strong force", proposing that the protons and neutrons in a nucleus were impermanent: a neutron could pass an electron to a proton, with the neutron then becoming a proton and the reverse. This process would be going on at a great rate at all times, and the net effect would be a strong attractive force. There were problems with Heisenberg's model, and few were enthusiastic about it.
The shy, brilliant Japanese physicist Yukawa Hideki (1907:1981) began to tinker with the notion that the strong force might involve an exchange of particles. He would eventually be vindicated by the discovery of the strong force "exchange particle", the "pion", with Yukawa receiving the Nobel Prize in 1949. In any case, the strong force was about a hundred times stronger than the electromagnetic force, but it only worked over short range.
BACK_TO_TOP* An understanding of the strong force led to an understanding of why nuclei became more unstable as they grew more massive, and why the ratio of neutrons to protons increased as well. The protons in a nucleus drove each other apart with the long-range electromagnetic force, while the neutrons held them together with the short-range strong force. As the number of protons increased, so did the "electromagnetic force couplings" AKA "Coulomb force couplings" between each proton and all the others. The strong force couplings only worked between neighboring particles, and so more neutrons were needed to keep the nucleus together. The balance between the long-range electromagnetic force and the short-range strong force became ever more fragile until, above a certain atomic number, it was impossible to form a stable nucleus. It was no coincidence that the strong force was about a hundred times stronger than the EM force, and that the heaviest stable nuclei had an atomic number of a little under a hundred.
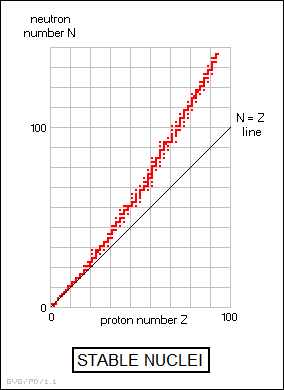
This is an intuitive way of looking at the issue, and in a domain as counterintuitive as quantum physics, relying strictly on intuition is a good way to walk into a booby trap. In the post-World War II period, Maria Goeppert-Mayer (1906:1972), a German physicist who came to the US and became an American citizen, came up with the idea that the particles in the nucleus occupied energy levels and energy shells, very much along the lines of those that are occupied by electrons in atoms. She and her colleague Hans Jensen (1907:1973) published a text on the concept in 1955, and the two shared the Nobel Prize in 1963.
The theory of nuclear energy levels is of course complicated, but a simplified model can illustrate how it relates to nuclear stability. Since both protons and neutrons are fermions, they both obey the Pauli exclusion principle, meaning that each level can accommodate only two particles, one with spin UP and the other with spin DOWN. Both protons and neutrons have their own energy levels; in this simplified model, the neutrons have equally-spaced energy levels, but due to the electromagnetic repulsion between protons the spacing between protons is greater:
proton neutron
< > < >
< >
< >
< >
< U >
< U D >
< U D > < U D > base energy level
This means that as the number of nucleons in a nucleus increases, the neutron energy levels tend to fill up more quickly than the proton energy levels. If the number of neutrons gets too far ahead of the number of protons, however, the nucleus is not at its lowest energy level and neutrons will tend to decay.
* The Ukrainian-American physicist George Gamow (1904:1968) came with an alternate theory in 1928, which became known as the "liquid drop" model of the nucleus. The basic concept is that the nucleons are like the molecules in a droplet of water, with the strong force acting as something like "surface tension" to keep the droplet together. Of course, nobody knew about the neutron in 1928, and so Gamow was not able to flesh out the idea properly; Niels Bohr hung on to it and developed it in later years, and Bohr is often incorrectly credited with coming up with the concept.
BACK_TO_TOP* While one faction of physicists discovered the neutron and the strong nuclear force, another faction was discovering a second nuclear force.
The story is also convoluted. The discovery of the neutron and the strong force did much to explain alpha decay, but it complicated finding an explanation of beta decay. In the days when the nucleus had been thought to be composed of protons and neutrons, beta decay seemed like merely like an electron escaping from the nucleus. The discovery of the neutron emphasized that there were no electrons in the nucleus, and so beta radiation was the result of the decay of the neutron into a proton and an electron.
To no surprise, this led to more puzzles. The first was that in beta decay, the kinetic energy of the proton and the electron produced in the breakdown of the neutron didn't seem to add up -- there was a deficit. This deficit had actually been noticed in beta decay before the discovery of the neutron. The deficit was credited to the emission of some unseen particle.
In 1934, the Italian physicist Enrico Fermi (1901:1954) published a detailed analysis of the expected properties of this mystery particle. When somebody wondered if it was actually the neutron, Fermi, who had an impish sense of humor, replied: "No, it's just a neutrino." -- implying something like a "baby neutron". The flippant name stuck. Of course it was neutral and massless, or nearly so, but Fermi also pointed out that it would be hard to detect even if someone was looking for it; the neutrino was able to zip through planets with very little likelihood of being stopped. There was skepticism over Fermi's claim, but the neutrino would finally be discovered in the 1950s.
* There remained the issue of why beta decay occurred. In 1933, Fermi managed to come up with a credible explanation, postulating a very short-range force that only operated on the distance scale of a single neutron. It was a very subtle force that didn't glue one particle to another so much as it glued a particle to itself, controlling beta decay. For this reason that physicists often prefer to semantically waffle and call it an "interaction" and not a "force".
Though it was originally called the "Fermi force", it became known as the "weak nuclear force" or just "weak force", because it was so much weaker than the strong force.
By the mid-1930s, the mysterious processes of radioactive decay were understood, at least in general. Alpha decay was the result of a tug-of-war between the electromagnetic and strong forces. Although a raw consideration of the relative balance of the forces indicated that the strong force should win far more often than it seemed to, George Gamow showed that adding in the concept of quantum-mechanical tunneling tipped the balance back towards the electromagnetic force. Beta decay involved considerations of the weak force.
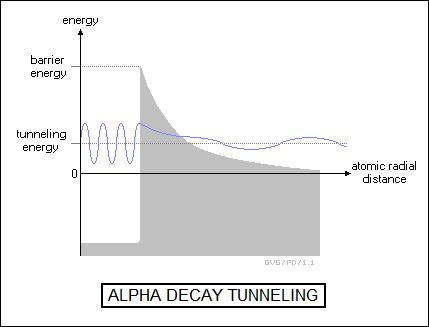
Both were entirely random processes: there was no "clock" inside the atom that said it would decay at some time or other, there was just a certain probability of it happening that resulted, in the scale of large numbers of radioactive atoms, in a highly specific half-life. The half-life was directly related to the probability of tunneling: over the long run, if there was a chance of the alpha particle escaping, it would do so eventually, with the average time required proportional to the tunneling probability.
BACK_TO_TOP