
* By the end of the 19th century, physicists had developed a reasonably consistent and detailed model of the Universe. They could look over their domain in well-deserved satisfaction, though there were still some nagging unanswered questions. Few had any suspicion that those nagging questions would turn many of the established concepts of physics on their heads.
* In the centuries since Isaac Newton more or less founded modern physics, his successors had elaborated on his concepts of a Universe that ran according to specific rules that could be described by mathematical formulas, allowing the behavior of that Universe to be predicted in a deterministic way. Classical mechanics, for example, had allowed the calculation of the orbits of the planets, reducing their movements to an elegant "celestial clockworks", and for nearly all practical purposes we use the same calculations today.
By the dawn of the 20th century, physicists had reason to feel pleased with their accomplishments, even if they generally understood they didn't actually know everything. Atomic theory had been invented by that time, though it was still a bit controversial, and most physicists believed that matter was composed of different kinds of atoms. 19th century physics and chemistry could really only tabulate the properties of those atoms, such as their weight, size, relative quantity, and inclination to bond and form molecules with other atoms -- as if physicists and chemists were zoologists tabulating data on animals in the field. There was certainly some sort of order involved, as demonstrated by the fact that atoms could be organized by their properties into the clearly structured chemical "periodic table". However, nobody had any real explanation of the reason for this underlying order.
One property of atoms was particularly puzzling: their methods of light emission. Classical physicists knew that matter emitted light in one of two ways. The first was known as "black body radiation". This phenomenon is obvious from general experience: heat a solid object, like a cannonball, and it will gradually glow dull red, then brighter red, then orange, then yellow, then white, then blue, then violet. These colors correspond to increasing frequency (or equivalently decreasing wavelength) of the light, or "electromagnetic (EM) radiation", emitted by the solid body.
Physicists also understood that at relatively low temperatures a solid body that would appear dark would still be emitting radiation, in the form of long wavelength "infra-red" EM radiation invisible to the human eye; and on the other extreme, as temperatures grew really high, a solid body that was radiating bright incandescent blue-violet radiation would emit an increasing amount of energy in the invisible "ultra-violet" short-wavelength EM radiation.
A "black body" is an idealized version of such a solid body, a perfect radiator that absorbs all energy and re-radiates it, losing no energy from reflection. Physicists were able to characterize the range of EM radiation emitted by a black body as a nice smooth "black body curve" in the form of a "hill", where the crest of the hill increased with temperature. The crest was in the infrared at low temperatures; at the colors red through violet as the temperature increased; and then in the ultraviolet as the temperatures grew really high. Incidentally, though it's not an important fact in the current discussion, the total energy emission of a black body rises with the fourth power of the temperature -- or in simpler terms, if the temperature is doubled, the total energy output increases by a factor of 16.
Black-body radiation seemed relatively straightforward, though it would not prove quite so simple when examined more closely. The other form of radiation, "line emission", was baffling on the face of it. A hot gas composed of a specific atom or molecule would emit EM radiation only at certain sets of specific wavelengths. Instead of the continuous rainbow spectrum produced by a black body, the gas produced a "line emission spectrum". If a continuous spectrum were shined through a gas, light would be absorbed at those same wavelengths, resulting in a set of dark lines on the bright continuous spectrum. These dark lines were called a "line absorption spectrum".
Different atoms or molecules had their own distinctive line spectra. Hydrogen, for example, had distinctive sets or "series" of spectral lines labeled "Lyman" (in the ultraviolet), "Balmer" (in the ultraviolet and visible ranges), and "Paschen", "Brackett", and "Pfund" (all in the infrared). There was a certain tidy arrangement in these series, since they could all be given by a neat formula, published in 1890 by a Swedish spectroscopist named Robert Johannes Rydberg (1854:1919):
wavelength = min_wavelength * ( N^2 / ( N^2 - series^2 ))
-- where "min_wavelength" is the minimum wavelength for each series, "series" is an integer value for each series, and "N" is an integer ranging from "series + 1" to infinity:
Lyman: series = 1 N = 2, 3, 4 ... Balmer: series = 2 N = 3, 4, 5 ... Paschen: series = 3 N = 4, 5, 6 ... Brackett: series = 4 N = 5, 6, 7 ... Pfund: series = 5 N = 6, 7, 8 ...
Even more interestingly, in many cases if two frequencies in each of these spectral series were added together, they gave another frequency in the group, a rule known as the "Ritz combination principle".
Line emission can be seen in gas discharge tubes, which are widely used in signs and public displays. In such tubes, an electric current is passed through a gas in a sealed tube, causing the gas to glow with a certain color. Neon gas, for example, glows orange. More significantly, at least as far as physicists were concerned, line spectra could be used as a "fingerprint" to determine the atom or molecule responsible for the line emission or absorption.
Physicists had been able to characterize the line spectra of many atoms and molecules, and had been able to perform such remarkable feats as determining the composition of distant stars using such line spectra. Nobody had any idea of any underlying reason why atoms and molecules had their own specific line spectra, however, or understood the significance of the orderly patterns in the line spectra of hydrogen. It seemed as though the atoms and molecules were like "resonant chambers" for light that could accommodate certain wavelengths but not others, or put another way like a musical instrument designed to play specific sets of notes. For the moment, that was as far as the thought went.
BACK_TO_TOP* As it turned out, it was black-body radiation, not line emission and absorption, that was the first key in the lock that opened the door to a new physics.
In the 1860s, the brilliant Scots physicist James Clerk Maxwell (1831:1879 -- "Clerk" was pronounced "Clark", by the way) published a series of papers in which he provided the basic equations that defined the behavior of electric and magnetic fields. One of the implications of "Maxwell's equations" was that a moving (or equivalently, oscillating) charged particle would produce a propagating electromagnetic field, in the form of visible light or some other EM radiation. Maxwell's equations neatly unified electricity, magnetism, and light -- but as is often the case with such great steps forward, they opened a can of difficulties as well.
Analysis of the black body spectrum using the idea that a black body was composed of oscillating emitters proved to be a particular difficulty. If the black body was emitting a true continuous spectrum of radiation, then radiation would be emitted at an infinite range of frequencies, with increasing energy as the frequency grew higher. Using this model, the energy of the emission of any black body would be infinite. Light a match, and the whole Universe would go up in a flash. This discrepancy from the observed behavior of a black body was called the "ultraviolet catastrophe".
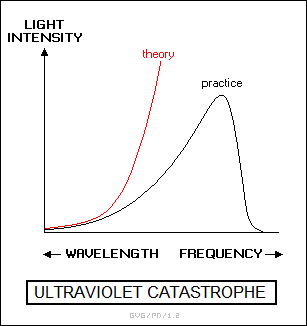
That was obviously not how things really worked. A German physicist named Max Planck (1858:1947) performed a theoretical analysis of the behavior of a black body to see if he could get something more in line with reality. After struggling with the exercise for some time, he came up with a simple assumption that allowed him to properly duplicate the black-body spectrum. He assumed that the black body was made up of "oscillating emitters". These oscillating emitters could actually only operate at energies in discrete steps, as defined by the formula:
energy = N * Planck's_constant * frequency = N * h * f
-- where "N" is a positive integer, and "Planck's constant" or "h" has the value:
6.626E-34 joule-seconds
Planck's constant is a very important value that will be seen again and again in this document. There is a related constant, known as "hbar" since it's usually written as an "h" with a crossline through the top, that is also heavily used, and is simply Planck's constant divided by 2PI:
hbar = h / 2PI
Essentially, Planck assumed that the energy produced was "quantized", or organized in steps instead of a continuum, and so he called these discrete increments of energy "quanta". He announced his discovery at a meeting of the German Physical Society in 1900. His findings created no particular controversy.
Planck was not really a visionary, being given more to plodding methodicalness than to brilliant leaps of intuition, and he regarded his "quanta" as just an arbitrary assumption that worked. The concept was "an act of desperation" as he put it, one that raised more questions than it answered. Planck did not realize at the time that he had just begun a revolution in physics. That would be realized later, and Planck would get the Nobel Prize in physics for his work in 1918.
BACK_TO_TOP* In 1905, Albert Einstein (1879:1955) published three significant scientific papers. Einstein had acquired a degree in physics but had not been able to find an academic position, so he was working as a technical examiner in a Swiss patent office to make ends meet. Einstein liked gadgetry and found the work amusing, and since it was an undemanding job, it allowed him to think about physics in his free time.
The first paper was on "Brownian motion", the random jostling of pollen and other very small particles when observed in solution under a microscope. Einstein performed a statistical analysis that showed the motion was due to the random thermal motions of the atoms around the particles. This seems like a trivial and obvious matter now, but Einstein's calculations matched observation so well that they provided a highly persuasive proof of the existence of atoms, helping to make believers out of die-hards who had remained skeptical of atomic theory.
The paper on Brownian motion was a significant contribution to classical physics. The other two papers opened doors in two directions to new domains in physics. The second of the three papers was on "special relativity", which Einstein had devised to explain a discrepancy in an optical experiment conducted in 1887 by experimental physicist Albert Michelson (1852:1931) and his colleague E.W. Morley.
The Michelson-Morley experiment had been conducted to determine the Earth's velocity relative to the "luminiferous ether". Light had been known to be a wave phenomenon and not a particle phenomenon since the beginning of the 19th century through experiments on light interference. In particular, in 1801 an English polymath named Thomas Young (1773:1829) had demonstrated that if light were shined through two narrow slits arranged side-by-side onto a screen, it would produce bands of light and dark lines, which arose because the light waves from the slits either added up when they were in phase, or canceled out when they weren't. If light had been a particle phenomenon, the light would have simply been most intense directly behind the slits, since that was where the light particles would pile up, with the light fading away elsewhere.
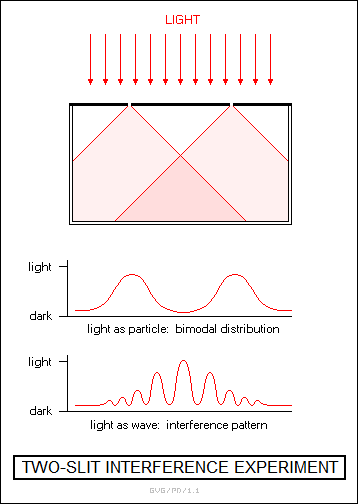
The discovery of the wave nature of light led to another puzzle. In classical physics, a wave phenomenon is a disturbance of a medium. For example, a water wave is a disruption of a body of water, and a sound wave is a disruption of the air. If light were a wave, then there had to be some medium to allow it to propagate. However, nobody could detect any such medium; if it existed, it was invisible. That was why some physicists had insisted that light had to be a particle phenomenon for a long time, but with the demonstration of interference effects they were forced to concede defeat. Very well, if light were a wave, then there had to be a medium to allow it to propagate. This medium was known as the "luminiferous (light bearing) ether"; it had to be invisible, and it had to permeate the entire Universe.
Light was known to propagate at a velocity very near 300,000,000 meters per second, and Maxwell's analysis showed that all EM radiation should have that velocity. If there was a luminiferous ether that filled the Universe, the Earth was moving through it, and the Earth's velocity should add to the velocity of light, if the Earth were moving against the ether; or subtract from it, if the Earth were moving with the ether. The Michelson-Morley experiment used an apparatus called an "interferometer" that measured interference effects in a light beam split to travel at right angles and then recombined. A difference in velocity between the light traveling along the two different paths would be measurable through interference effects of the two beams.
The experiment gave negative results: there was no difference in velocity between the two beams. Further experiments only confirmed this negative result. Einstein's theory of relativity basically accepted the result as a given and outlined the implications. These implications are subtle, are not entirely relevant to the current topic, and are discussed elsewhere, and so they are simply summarized here.
The theory of special relativity stated that the speed of light was an absolute constant, meaning that no matter how fast an observer was moving away from or towards a source of light, the observer would measure the speed of light as exactly the same. As a consequence of this ridiculous-sounding, but experimentally provable, postulate, it could be shown that:
At the speed of light, time would come to a stop, the length of the object would be zero, and its mass would be infinite.
For current purposes, special relativity had two significant results. The first was that it helped undermine the notion of the luminiferous ether. Did light really propagate through a medium? The brilliant English scientist Michael Faraday (1791:1867), relying on his astonishing intuition, had suggested decades before that the ether was a useless concept and that light might very well propagate without a medium. The idea had gone nowhere at the time, since nobody could imagine how such a thing could be. It wasn't any easier to swallow in 1905, but the idea was becoming much harder to ignore.
The other implication was that the increase of mass of an object as it expended energy to accelerate faster and faster demonstrated that mass and energy were equivalent, as shown by the most famous equation in all of science, E = MC^2, or:
energy = mass * speed_of_light^2 = m * c^2
This was actually produced in a fourth, relatively minor, 1906 paper, and not really explained clearly until 1907. Since an object moving at the speed of light would have infinite mass, it would take infinite energy to get it there, which reaffirmed the difficulty of reaching that speed.
* It is the third of Einstein's 1905 papers, on the "photoelectric effect", which is most important here. As mentioned, physicists and chemists more or less understood atomic theory at the beginning of the century. They also understood the existence of a subatomic particle, the "electron", which had a negative electric charge and made up an electric current. Electrons could be produced by stripping them from atoms, leaving behind positively-charged "ionized" atoms.
From 1879, the British physicist William Crookes (1832:1919) had conducted studies of faint glows in "discharge tubes", which were long cylindrical glass tubes that had a negative electrode or "cathode" at one end and a positive electrode or "anode" at the other, and which were partly evacuated. An electric current flowing between the two electrodes generated dim colorful light, and Crookes found the phenomenon fascinating. The "Crookes tube" was improved by widening the end with the positive electrode, coating that end internally with a layer of "phosphor" material such as zinc sulfide (ZnS) that emitted light, or "phosphoresced", when stimulated by electricity, and by being much more thoroughly evacuated.
Surprisingly, an electric current continued to flow even when there didn't seem to be any gas in the tube. The mysterious electrical flow was given the name of "cathode rays", of course making the improved Crookes tube a "cathode ray tube" -- which in turn would be the direct ancestor of the TV picture tube used in the 20th century. The cathode rays were assumed to be some form of light.
In 1897, the British physicist Sir Joseph John "J.J." Thomson (1856:1940) of Cambridge University in the UK was able to show that the radiation was in the form of very small negatively charged particles that could be deflected by electric or magnetic fields. The particles became known as electrons, and he was able to determine the ratio of charge to mass, using a cathode ray tube.
Thomson's experimental apparatus involved placing a magnetic yoke around the neck of the tube, with the magnetic field cutting across the tube, and also placing electric plates on the top and bottom of the tube. Electrons would "boil" off a heated filament at one end of the tube, to be drawn down the tube by a positively-charged "focusing anode" in the shape of a flat can with a hole through them middle. Some of the electrons would fly through the hole and then go on to strike the zinc sulfide phosphor screen at the end of the tube.
An electron moving through the magnetic field was deflected downward on its way to the screen. The plates were then activated, and the voltage measured at which the electron beam ran straight down the tube, to hit the screen in the center. For a given "nulling voltage", a more massive electron would result in a smaller deflection when the nulling voltage was removed. A few simple calculations gave the charge-mass ratio.
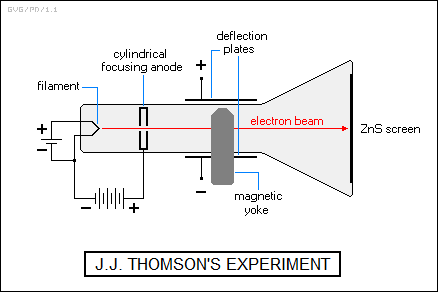
Thomson had no way to measure the mass of the electron, but it was obviously much less than that of a hydrogen atom, the lightest possible atom. Electrons were shown to be associated with atoms, with their removal leaving the atoms positively charged. They were the first subatomic particles to be discovered. Thomson received the Nobel Prize for physics in 1906 for this work. Incidentally, Thomson was not actually the first to notice the electron, but he was the first to determine anything about its properties, and so he remains credited with its discovery.
* Another revelation about electrons had been uncovered by a different electrical device, the "phototube". This featured an anode and a "photocathode" that emitted electrons when hit by light. This "photoelectric effect" had been discovered by German physicist Heinrich Hertz (1857:1894) in 1887.
There was nothing in the photoelectric effect that seemed too surprising until it was investigated in more detail by Thomson and, independently, a Czech-German researcher named Philipp Lenard (1862:1947). A careful analysis showed that the energy of the "photoelectrons" produced was not dependent on the intensity of the light shined on the photocathode. No matter how bright the light was, the energy of the electrons would not exceed a certain level, though the flow of photoelectrons would increase. However, if the frequency of the light increased, the maximum energy of the photoelectrons would increase along with it. On the other hand, below a certain frequency there was no photoelectron emission at all, no matter how intense the light was.
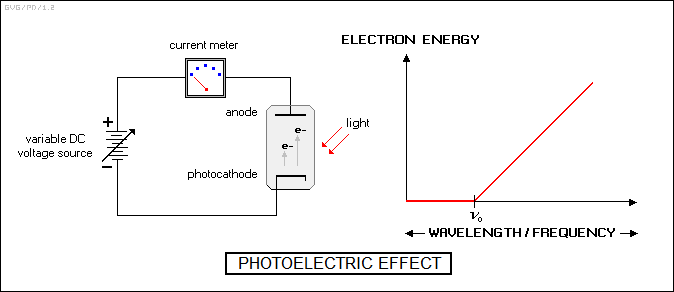
According to classical physics, if the photocathode simply soaked up light energy, then sooner or later, depending on the intensity of the light, it would accumulate enough energy to cause photoemission. That wasn't the case. If the light was below a certain frequency, it could be shined on a photocathode until Judgement Day and no electrons would be produced. To really confuse matters, if the light was above that wavelength, photoelectrons would be produced immediately, with no time delay linked to the intensity of the light. Lenard won the Nobel Prize in 1905 for his work.
Einstein's third paper provided an explanation of the photoelectric effect. He took as his starting point Planck's notion that the oscillators that produced black body radiation operated in quantized energy levels, and went a step further to suggest that light itself was produced in quanta as well, with the energy of these light quanta given by:
energy = Planck's_constant * frequency = h * f
Such light quanta would be named "photons" by the prominent American chemist Gilbert N. Lewis (1875:1946) in 1926. Einstein's model neatly explained the photoelectric effect. Suppose that it took a certain amount of energy to free an electron from the photocathode, with this energy given as the "work function" or "W". If the photon energy was less than the work function, the photon would never pry an electron loose from the photocathode. If the photon energy was more than the work function, it would cause the immediate emission of an electron with the energy:
electron_energy = W - h * f
Maxwell had showed that an electromagnetic wave had the momentum:
momentum = energy / speed_of_light = e / c
-- and so a photon had the momentum:
momentum = h * f / c = h / wavelength
In other words, the shorter the wavelength of the photon, the greater the momentum of the photon. Einstein's considerations also demonstrated that a photon had a rest mass of zero, but his theory of relativity had demonstrated that it was impossible to bring a photon to a halt, or even affect its velocity in any real way. Since his theory of relativity had also more or less disposed of the ether wind, Einstein's postulate that light had particle-like aspects helped explain how it could propagate without a medium. Another implication, specifically that a field (in this case, the electromagnetic field) could be based on the interactions of particles (in this case, photons), wouldn't be exploited for decades.
* Einstein's explanation of the photoelectric effect also explained a related process, the production of "X-rays". In 1895, the German researcher Wilhelm Conrad Roentgen (1845:1923) discovered that a cathode ray tube emitted an invisible form of radiation that could penetrate through soft materials but not hard materials. He called it "X radiation" because he didn't know what it was, though it would later prove to be high-energy electromagnetic radiation. J.J. Thomson found X-rays very interesting since they could make the air electrically conductive, or ionize it; when Thomson discovered the electron in the next year, he realized the X-rays were knocking the electrons out of the gas atoms.
Roentgen won the 1901 Nobel Prize in physics for his discovery. Although he didn't know what X-rays really were, he did know that they could sleet right through the body onto a photographic plate, leaving "shadows" of the heavier bones on the image. The first formal X-ray photograph was of the hand of Roentgen's wife Bertha, showing the bones in her hand and her wedding ring.
Within a few years, medical X-ray photographic systems were on the market. There were also X-ray systems that used fluorescent plates to give "real-time" images of bones and the like, but these "fluoroscopes" were little more than a gimmick. There was an X-ray craze for a time, with a certain amount of public hysteria over the notion that X-ray machines could be used to see through walls and into houses. That was nonsense, but X-rays did turn out to pose a real threat: it would take decades and many tragedies before everyone recognized that overexposure to X-rays could cause cancers, and even burns down into the bones.
X-ray imaging of course still remains in widespread use, though using much greater precautions. Although Roentgen used a gas-filled tube to generate X-rays, modern X-ray machines use variations on the "Coolidge" tube, invented by the American physicist William David Coolidge in 1913, which can generate more effective "hard" X-rays. The Coolidge tube is a sealed, evacuated metal tube with a window on one side. The negative cathode is curved to focus electrons boiling off of it onto an anode made of molybdenum or some other heavy metal raised to a high relative positive electrical potential. The anode is at a 45-degree angle to the electron flow, allowing the X-rays produced by the electrons colliding with the anode to flow out the window.
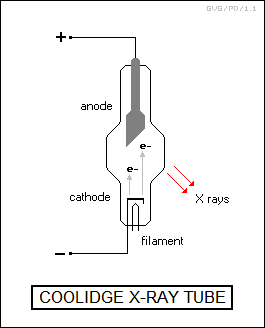
An electron boiling off the cathode flies across the tube and smashes into the anode at high velocity and energy, and in its rapid deceleration it gives up this energy, emitting an X-ray photon. This process, known by the German term of "Brehmsstrahlung (braking radiation)", is the inverse of the photoelectric effect, which involves a photon transferring its energy to an electron.
* Einstein himself said that of his three 1905 papers, only the one on the photoelectric effect was "truly revolutionary". The paper on Brownian motion was strictly classical physics, and the theory of special relativity, though groundbreaking, was really an extension of classical physics. The paper on the photoelectric effect, in contrast, was a step off the edge of the world, and Einstein had taken that step reluctantly. His notions were not widely accepted at the time, since they implied that light acted like a particle in some respects and not a wave, when it was provably a wave. There was the particular sticking point at the time of the paper's publication in that there was no real experimental proof to back it up. Although Max Planck tended towards the conservative, he became one of the believers, writing perceptively in 1910 that if Einstein's theory of the photoelectric effect were to be proven true, "as I expect it will, he will be considered the Copernicus of the 20th century."
In 1915, the American experimental physicist Robert Millikan (1868:1953) released the results of experiments to check on Einstein's theory. Millikan was an ingenious and extremely precise experimentalist, having already conducted a landmark experiment, its results published in 1909, in which he had determined the charge of the electron by finely atomizing oil droplets, stripping them of electrons or "ionizing" them with X-rays, and injecting them between two charged electric plates. He then observed their rates of acceleration through a microscope, allowing him to obtain an estimate of their charge. Since J.J. Thomson had already determined the electron's charge-mass ratio, that gave the mass of the electron, which turned out to be about 9.1E-31 kilograms.
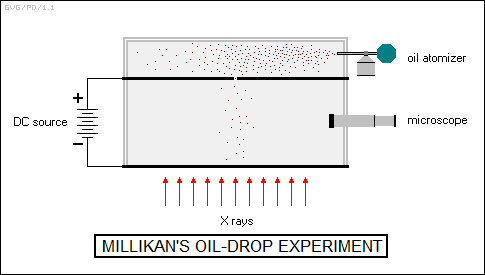
Millikan's studies of the photoelectric effect were just as exacting and confirmed Einstein's theory. Few disputed his results, since his oil-drop experiments had given him substantial credibility. The irony was that he had actually been trying to prove Einstein wrong, and even though Millikan was not noted for flexibility in his views, he had finally been forced to admit defeat: "I spent ten years of my life trying to disprove that 1905 equation of Einstein's, and contrary to all my expectations, I was compelled in 1915 to assert its unambiguous verification in spite of its unreasonableness."
Einstein himself admitted the concept had its strongly unreasonable aspects, stating in 1911: "I insist on the provisional nature of this concept, which does not seem reconcilable with the experimentally verified consequences of wave theory." Even 40 years later, in 1951, he wrote a friend: "All these 50 years of conscious brooding have brought me no nearer to the answer to the question: 'What are light quanta?' Nowdays every Tom, Dick, and Harry thinks he knows it, but he is mistaken."
Although Einstein is best known for relativistic physics, it was his paper on the photoelectric effect that won him the Nobel Prize in 1921. Relativistic physics was still controversial enough at the time that the Nobel Prize committee made a specific point of refusing to consider it; however, as has been discussed, it is arguable that his work on relativity was more significant than his "discovery" of the photon. Incidentally, Millikan won the Nobel Prize in 1923 for his oil drop experiments and confirmation of the photon.
* Further proof that light had particle-like properties was provided in 1923 when an American experimental physicist named Arthur Holly Compton (1892:1962) bounced X-rays off a block of graphite, or more specifically bounced them off the electrons in the carbon atoms that made up the block of graphite. By classical electromagnetic theory, the electrons should have simply absorbed the EM energy and re-radiated it at the same wavelength. However, according to photon theory, the collision between the photon and the electron should result in an exchange of momentum, just as if it were a collision between two particles, and the change in momentum of the photon would result in a change in its wavelength.
Compton's experiments gave the changes in wavelength expected in theory. Although a Dutchman named Peter J. Debye (1884:1966) independently performed the same experiment in parallel with Compton, the scattering of photons by electrons is known as the "Compton effect" or "Compton scattering." This experiment helped convince die-hards that light did have some properties of a particle.
It did not, however, make even the believers happy with the idea. How could something be a wave and a particle at the same time? From the point of classical physics, that was a complete contradiction, pure ridiculous doubletalk, like saying something was round and square at the same time. At the time, there were other lines of research that were moving towards a resolution of this contradiction, but the resolution would not really make things feel much tidier. Einstein himself would ultimately decide that he'd had enough and refuse to have anything more to do with it. Einstein's discoveries were revolutionary, and if he had embraced revolution with his ideas, they would still go farther than even he was comfortable with. The English poet Alexander Pope (1688:1744) once praised Isaac Newton with the verse:
Nature and Nature's law lay hid in night. God said "Let Newton be" and all was light.
Much later, another English poet, Sir John Squire (1882:1958), added:
It did not last; the Devil, shouting "Ho! Let Einstein be!" restored the status quo.BACK_TO_TOP
* While Max Planck was explaining blackbody radiation and Einstein was explaining the photoelectric effect, other physicists were learning more about the atom. In 1896, the French physicist Anton Henri Becquerel (1852:1908), having heard about Roentgen's discovery of X-rays, wondered if phosphorescent materials also emitted invisible radiation. He tried placing phosphorescent materials on top of photographic plates wrapped in thick black paper. Nothing happened until he tried uranyl sulfate, which fogged the photographic film.
That suggested that some phosphorescent phenomena produced X-rays. However, phosphorescent materials by definition only glow after exposure to light or other energy. Becquerel then stashed some of the uranyl sulfate crystals in a drawer with photographic plates while he worked on other things, and when he came back to these materials a week later, he got curious and developed the photographic plates. They were fogged; the phosphorescence as such had nothing to do with the mysterious radiation emitted by uranyl sulfate. Becquerel had discovered "radioactivity", though he only knew that it happened and had little concept of the details. He believed that the radioactive material spontaneously emitted X-rays, which was true, but as he probed further, he began to realize that wasn't all there was to the story. It was still a mind-boggling concept to think that materials would produce such high-energy radiation on their own, without the slightest prompting.
Two French chemists, the husband and wife team of Pierre Curie (1859:1906) and Polish-born Marie Curie (1867:1934), were able to demonstrate that radioactivity was a property of several types of atoms. They investigated an ore known as "pitchblende" that was more radioactive than the uranium salts studied by Becquerel and isolated two radioactive elements, "polonium" (named in honor of Poland) and "radium". Radium was about a million times more radioactive than uranyl sulfate.
The Curies determined that the "decay" of radium produced heating effects that cumulatively provided far more energy than could be provided by burning an equivalent mass of, say, coal. The prospect of finding a new energy source made the study of radioactivity just that more interesting; Einstein's later discovery of mass-energy equivalence would suggest to many physicists that the amount of energy might be staggering, though for the time being nobody had much of a clue as to how that energy might be seriously tapped. Becquerel and the Curies would share the 1903 Nobel Prize in physics for the discovery of radiation.
Further radioactive elements were discovered, including "thorium" and "actinium". In 1897:1898, the New Zealander physicist Ernest Rutherford (1871:1937), working in J.J. Thomson's Cavendish laboratory at Cambridge, conducted observations of radium and thorium. Rutherford was using a simple device called an "electroscope" to check for radiation. There were different forms, but the most familiar variant looked like a sealed box with a window in the side and a metal probe fitted through the top via an insulating washer. The probe ended with two little gold leaves. If an electric charge were transferred to the probe, the two leave would bend apart. Radiation flying through the box would ionize the air inside, which allowed the charge to flow away from the gold leaves, causing them to relax back together. The level of radiation could be measured by timing how long it took for the leaves to fall back together.
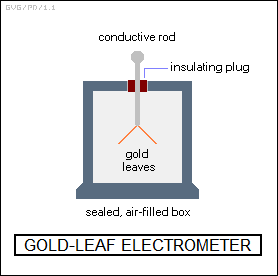
Incidentally, there was also a somewhat more sophisticated variation on the instrument called the "electrometer" that featured, say, twin metalized quartz fibers and a built-in microscope with a graticule. The distance of separation of the fibers could be measured to indicate the strength of the electric charge.
Rutherford's experiment involved covering a sample of radioactive uranium with sheets of aluminum foil, then measuring the level of radiation. The radiation kept going down steadily as more sheets were added, but Rutherford finally reached a point where the radiation leveled off and didn't seem to fall any more. It turned out it would fall if a considerably heavier amount of shielding was added. The conclusion was that there were two kinds of radiation being emitted by the radioactive sample -- one that was easily blocked, which he called "alpha" radiation, and one that wasn't so easily blocked, which he called "beta" radiation.
The beta particles, as it turned out, behaved exactly as did J.J. Thomson's electrons, and so were correctly judged to be electrons. For the moment, the alpha particles remained a mystery. To complicate matters further, studies published in 1900 by a Frenchman named Paul V. Villiard (1860:1934), in which he subjected the radiation to electric and magnetic fields, also revealed a third type of radiation, known as "gamma" particles. They had no charge and so were not affected by electric or magnetic fields; they were also substantially more penetrating than beta particles. Gamma rays would turn out to be very high energy EM radiation, even more energetic than X-rays.
In 1899, Rutherford went to Canada to take a professorship at McGill University. His observations there of the radioactivity of uranium showed that the energy released was much greater than could be obtained from any chemical process. In 1900, he suggested that the radiation might be caused by some alteration in the internal structure of an atom, though at the time it wasn't generally thought atoms had much of an internal structure.
Rutherford also performed observations of radioactive thorium at the time, which gave puzzling results: the intensity of the radiation seemed to fluctuate, in particular under breezy conditions, as if the radiation was being affected by the wind. Further work led him to believe that thorium was emitting a radioactive gas that was blown off by the wind. Since he lacked the skills to perform a chemical analysis to track down the gas, he called in a British chemist, Frederick Soddy (1877:1956), to help him with the effort.
The investigations of the two showed that, in emitting radiation, thorium produced a new element, which they originally called "thorium-X" but which proved to be radium. Radium in turn decayed radioactively to a radioactive gas, which they called "radon". They had discovered transmutation of the elements, in which an element broke down into another, lighter element, releasing alpha, beta, or gamma radiation in the process.
That was a big conceptual step forward, since atoms had been considered to be fundamental and unchanging up to that time, and Rutherford joked with Soddy that they might be accused of being alchemists. Rutherford's work won him the Nobel Prize in 1908 -- though to his amusement it was the prize for chemistry, in essence accusing him of being a chemist.
BACK_TO_TOP* Rutherford had returned to the UK in 1907 to take a professorship at the University of Manchester. In 1909, he placed some radioactive material inside a double-walled glass vessel, with a thin inner wall, a thick outer wall, and a vacuum between the two walls. The radioactive material was known to emit alpha particles, which would penetrate the thin inner wall but not the thick outer wall. After a few days of accumulation of alpha particles in the space, helium was detected by spectroscopic analysis, leading to the conclusion that alpha particles were helium atoms stripped of electrons, Apparently, in some obscure way, the radioactive atoms were made up at least partly of helium atoms, with their loss leading to radioactive transmutation of the atom.
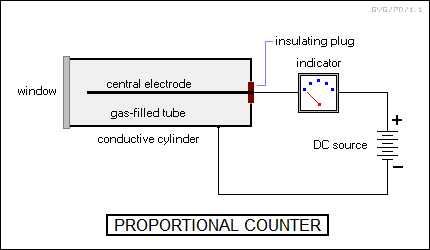
To probe further, Rutherford needed better instruments. He helped his assistant develop Hans Wilhelm Geiger (1882:1945) design an instrument to detect radiation through the ionization of gas in a sealed tube by the passage of a particle. A decade later, Geiger would work with Walther Mueller (1905:1979) to refine the device, which became known as the "Geiger-Mueller counter", or the "Geiger counter", or more generally a "proportional counter". In its mature form, it consisted of a gas-filled tube with metal walls and a wire electrode running down the middle, with a high electric potential between the walls and the wire. A charged particle passing through the tube would ionize the gas, allowing electricity to flow, completing the circuit, and kicking an indicator needle or emitting a "click" through a loudspeaker. The potential across the tube was great enough to ensure that even a single particle would cause the breakdown of the gas.
Rutherford and his team went on to make another important discovery about the atom. J.J. Thomson, in his discovery of the electron, had suggested that an atom was basically a positively-charged ball of uniform density that included a smattering of electrons distributed more or less at random through its interior, with the result that the atom normally had a neutral electric charge.
Thomson's "plum pudding" model, as it was called, was a reasonable first approximation. Rutherford decided to test it. According to the Thomson model, an alpha particle passing through an atom should be deflected by a certain small amount. The electroscope that Rutherford had used in previous experiments couldn't detect a single alpha particle, but German researchers had come up with a scheme that could, using a zinc sulfide phosphorescent plate observed through a microscope that produced "scintillations" or spots of light when struck by an alpha particle. The plate assembly could be moved around the target along a ring to determine the scattering at different angles. It was a laborious, tiring procedure, but it turned out to do the trick.
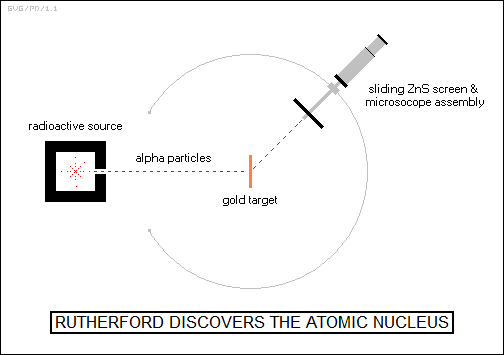
In 1910, Rutherford had his assistants Geiger and Ernest Marsden (1889:1970) bombard a gold foil with alpha particles from a radioactive source to see if the scattering pattern, as revealed by the observations of scintillations. It didn't. In fact, about 1 in 20,000 alpha particles bounced back from the atom. Rutherford was shocked, famously writing: "It was quite the most incredible event that ever happened to me in my life. It was as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
Obviously, the Thomson model was not correct, and a few years later Rutherford would simply describe it as "not worth a damn." In May 1911, after puzzling over the matter for months, Rutherford published his conclusions, asserting that the real state of affairs was that the mass and positive charge of the atom was actually concentrated in a tiny core or "nucleus" of the atom, with the electrons orbiting around it. It was only this dense central nucleus that could have the mass to force alpha particles to bounce backward, with the compactness of the nucleus also demonstrated by the fact that the bounces took place so rarely.
The idea was not entirely new: the Japanese physicist Nagaoka Hantaro (1865:1950) had suggested the nuclear atom in 1903, but his thoughts on the matter had been purely speculative, not backed up by detailed observations, and suffered from a number of logical defects. Although Nagaoka had visited Rutherford early in 1911, they hadn't discussed atomic models and Rutherford had no idea of Nagaoka's speculations along that line; when he was told, Rutherford, a scrupulous man, added a note to his article, crediting Nagaoka's priority.
With the discovery of the nuclear model, alpha particles were then recognized as the nuclei of helium atoms. The exact structure of that nucleus remained a mystery for the moment, but Rutherford and his people were working on that. He suspected that the nucleus might be made up of charged particles, each with a positive charge that matched the negative charge of each electron, but which were much heavier than the electron. Gold atoms were heavy and had a highly-charged nucleus that strongly repelled the alpha particles; obviously, if he wanted to probe the nucleus more closely, he needed to use a lighter element, and what lighter element was there than hydrogen gas?
Rutherford and Marsden began to conduct experiments with a variation on the scintillation observations, replacing the gold foil with hydrogen gas. Calculations showed that the alpha particles should be increasingly damped out by the gas as their distance of travel through the gas increased, until at a certain critical distance no alpha particles got through at all. However, even beyond that critical distance, some scintillations were detected, and continued to be detected at up to four times the critical distance.
Applying a magnetic field showed the particles were positively charged; Rutherford suggested that the particle was the nucleus of the hydrogen atom, since it had almost exactly the same mass as the hydrogen atom. The alpha particles, it seemed, would hit the nucleus of hydrogen atoms and knock the atoms to pieces, sending the nucleus flying. Rutherford initially called the particle the "H particle".
Further experiments were needed to pin the idea down, but with the beginning of World War I, pure research went on the back burner. Geiger went home to Germany to prevent from being interned as an enemy alien, Marsden went back to New Zealand, and Rutherford ended up working on investigation boards for the British admiralty, though he managed to conduct some experiments in his spare time. He managed to produce H particles by bombarding boron, sodium, fluorine, aluminum, phosphorus, and nitrogen with alpha particles.
J.J. Thomson retired in 1918 and Rutherford, by then one of the world's most famous experimental physicists, was hired in 1919 to take his place at the head of the Cambridge Cavendish laboratory. The war was now over, and it was time to go back to probing the atom again. By this time, Rutherford was increasingly confident that he had discovered a building block of the nucleus, a heavy and positively-charged counterpart to the electron. He announced his findings to the world in 1919, naming the new particle the "proton", from the Greek word for "first". He suggested that the nuclei of heavier atoms were made up of protons. The proton would be measured to be 1,836.11 times larger than the electron, with a weight of 1.6726E-27 kilograms.
The Rutherford atom, then, consisted of a set of electrons orbiting around a nucleus made up of protons. The arrangement looked something like a miniature solar system, with the nucleus something like the Sun and the much smaller electrons like planets orbiting the Sun, and this notion would lead in later decades to science-fiction stories that suggested they really were solar systems in miniature. However, by that time such an idea was complete nonsense. Rutherford's nuclear atom was a good second approximation, but the truth that would follow it was far more devious than any science-fiction story could have described.
