
* Rubidium is a member of the alkali metals family:
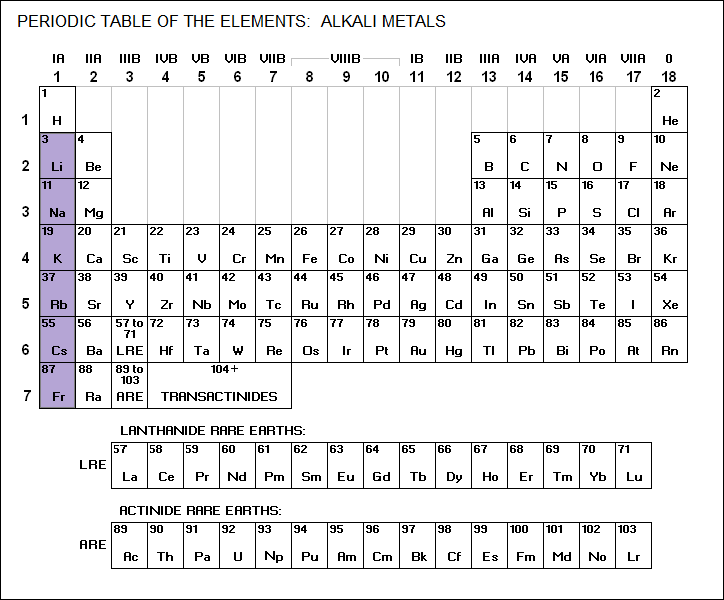
____________________________________________________________________
RUBIDIUM / Rb / 37
A soft, white metal that is so highly reactive that it will not
only ignite in water, but even in air and with ice. Two isotopes
are found in nature: Rb<85/37> (72%), which is stable, and
Rb<87/37>, which is radioactive, with a half-life of 50 billion years.
atomic weight: 85.4678
abundance: 22nd
density: 1.532 gm/cc
melting point: 38.89 C
boiling point: 686 C
valence: 1 2 3 4
____________________________________________________________________
Rubidium has no serious commercial applications. Anything that could be done with it can almost always be done by sodium or potassium, which are more common. As a result, nobody makes much effort to refine rubidium, and such as is produced for lab studies is preposterously expensive.
