
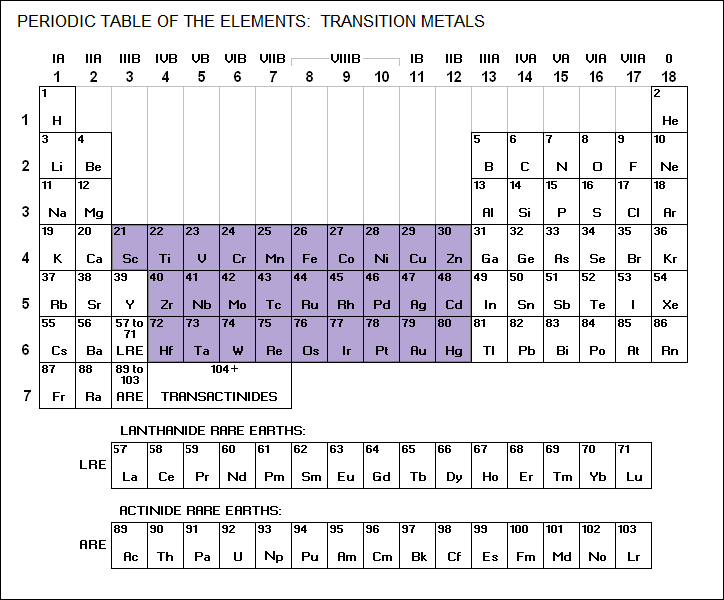
* Iron is a member of the transition metals family:
____________________________________________________________________
IRON / Fe / 26
Pure iron is a silvery-gray, soft metal that rusts in damp air or
water, and dissolves in acids. Its symbol Fe is from "ferrum", the
Latin name for iron. Four isotopes occur in nature:
Fe<56/26> / 92%
Fe<54/26> / 6%
Fe<57/26> / 2%
Fe<58/26> / 0.3%
All are stable.
atomic weight: 55.845
abundance: 4th
density: 7.874 gm/cc
melting point: 1,535 C
boiling point: 2,750 C
valence: 2 <3> 4 6
____________________________________________________________________
The "Fe++" ion was once known as the "ferrous" ion while the "Fe+++" was known as the "ferric" ion; they are now known as "iron(II)" and "iron(III)" respectively. Iron is the most common and almost unarguably the most important metal, produced in hundreds of millions of tonnes yearly to build cans, machines, structural elements, and so on. It is an important element in our biological processes, being the core of the hemoglobin molecule in our blood.
Iron is usually alloyed with carbon to harden it, creating steel, though high concentrations of carbon make the alloy brittle, resulting in what is confusingly referred to as "pig iron". It can be alloyed with other metals to provide a range of characteristics.
