
* From the middle of the 20th century, the understanding of the molecular basis for heredity provided evolutionary biology with powerful tools that Darwin himself couldn't have imagined. We now understand how mutations support MET, and have identified sets of genes, the "immortal genes", that are found in widely unrelated organisms, providing a strong basis for the notion of common descent.
* As noted, the biggest weakness of THE ORIGIN OF THE SPECIES was Darwin's ignorance of heredity, and he never learned of Mendel's discoveries in genetics. Now we understand the mechanics of heredity down to the molecular level of DNA -- and it provides a central tool for MET.
As discussed previously, the primary function of DNA is to code the production of proteins, using triple combinations of the three nucleotide "bases" -- A, T, C, G. A gene consists basically of a sequence of such triplets, beginning with a START code and ending with a STOP code. The code is effectively universal among all organisms, providing strong evidence of the commonality of all life; there are, however, small variations in the code, with some species using slightly different codes than others and the variations following known taxonomy.
The complete genomes of different organisms also follow the taxonomic tree of life, with small variations among closely related organisms and large variations between distantly related organisms. The coding of the genome can be regarded, for purposes of analysis, as something like a "serial number" -- a very long serial number -- that is passed down through lines of descent, with occasional changes in the serials from generation to generation. The similarity of these serials can be used to establish family relationships between different species: two species with similar serials are closely related, two species with dissimilar serials are not closely related. Relationships can be identified by the pattern of changes in the genome between one species and another.
There are a number of approaches to taxonomic analysis of DNA. One of the problems is that a genome is big. It's a fair amount of work to sequence the entire genome of an organism, and it's simply too laborious, as well as overkill, to completely sequence a large number of individuals. It is often more practical to focus on specific genetic sequences in the genome.
Mitochondrial DNA (mtDNA) is a popular target, because the mitochondrial genome is small and mitochondria are common in eukaryotic cells, meaning mtDNA is relatively easy to sequence. However, use of any one genetic sequence is a bit unreliable. Given the analogy of a genome as a long serial number, what happens if the serial in organism A gets changed by one value in its offspring B, but then gets changed back to the original value in the next generation, C? Analysis would seem to show that A and C belonged to the same generation. Similarly, the serials might end up being changed in exactly the same way by coincidence along two separated lines of descent.
Such misleading patterns are unlikely, of course, but they're not ruled out, and in practice sets of specific genetic sequences are used, with a "majority vote" scheme employed to weed out false matches. For example, a taxonomic analysis of the relationships between the members of the cat family used 30 different sequences, including mtDNA. Incidentally, the survey had some surprising findings, for example showing that cougars and cheetahs were very closely related, and that housecats were more closely related to cougars than they were to bobcats.
There are some "shortcuts" that can be used as well. One is based on the fact that the individual genes in our genome are not found in contiguous sequences of DNA. A gene is usually broken up into segments by chunks of "noncoding DNA" -- that is, DNA sequences that don't actually code for proteins -- known as "long interspersed elements (LINEs)" and "short interspersed elements (SINEs)" that don't code for proteins and are chopped out when the gene is interpreted into a protein by the cell. LINEs and SINEs are introduced into a genome at very rare instances over past history, and once inserted they remain in later generations. The pattern of LINEs and SINEs in a genome provides a very precise means of tracing the genealogy of a species without having to decode the complete genome. Noncoding DNA, incidentally, is an interesting if somewhat confusing subject, considered in more detail below.
With the power of DNA, relationships that were once obscure become clear. Is the panda bear really a bear, or is it more closely related to the little red panda, or the raccoon? DNA says it's a bear. DNA analysis has not only shown, from analysis of old feathers, that the dodo bird was a species of pigeon; it was also more closely related to certain existing species of pigeons than those species are related to some other species of pigeons. It is even possible to trace the ethnic derivations of humans from their DNA, a process that is potentially very useful in forensic investigations, though it is also controversial since it hints at "ethnic profiling".
Although genomic shortcuts are very handy tools, the drive remains to acquire whole genomes, with new genomes of organisms being produced at an accelerating rate -- and presenting a challenge to data storage and analysis. As we continue to unravel the genomes of more and more organisms, we are able to see patterns in those genomes of the past evolution of organisms, being able to identify gene changes that split one branch of organisms off from another, down to ever finer levels of detail. We are, in short, acquiring a map of the genetic changes that produced the organisms that exist today. Over the long run, we will not only be able to establish the relationships of organisms -- we will know the genetic changes that produced them as well.
BACK_TO_TOP* Differences in genomes will arise through processes of mutation. DNA is copied or "transcribed" with mechanisms that ensure high fidelity in the transcription process. Even a good typist has an error rate billions of times greater than the DNA transcription process. The transcription has to be that good simply because there are so many cells in our body, each with its own DNA, grown from the single-celled ovum that we each started out from. If the transcription process weren't almost perfect, we'd never be able to grow all those cells in working condition.
Mutations do occur in DNA, of course. DNA replication is going on all the time, and sometimes it goes wrong. The most common mutation is to simply switch one of the four bases -- A, C, G, or T. There are also mutations that can delete parts of a genome or add to a genome -- adding base pairs, an entire duplicated gene, entire duplicated chromosomes, or even entire duplicated genomes.
Duplicated genes are particularly important to evolution. They occur when the genome is replicated and the replication process "stutters", producing two copies of a gene in the output. The occurrence of gene duplication is rare, with the odds of the duplication of any specific human gene being in the range of one in a hundred to one in a thousand per million years. That is still enough to ensure that humans and other animals have plenty of duplicated genes. Initially, both copies of the duplicated gene work the same, but over time they can mutate into different forms with different effects through single-point mutations.
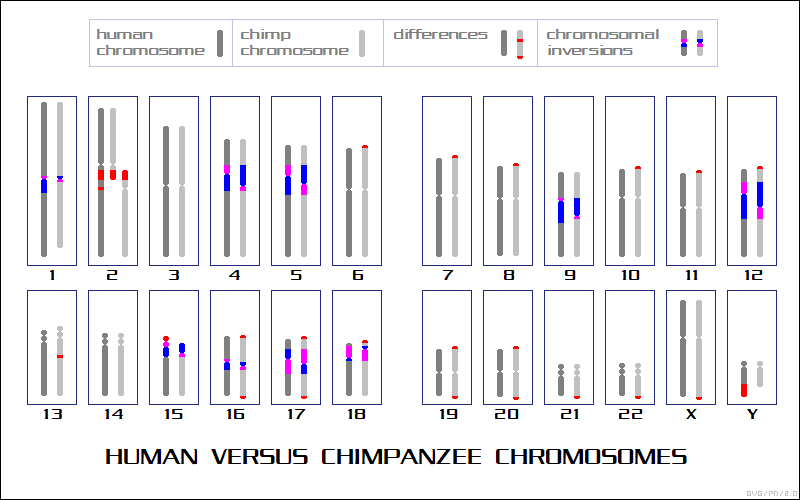
Chromosomal duplications in humans are known to tend towards the disastrous -- the form of mental impairment known as "Down's syndrome" is due to a chromosomal duplication -- but chromosomes can also split into two, or fuse together, with less likelihood of drastic consequences. In fact, it is now known that the reason humans have 23 sets of chromosomes, while the other apes have 24, is because one of our chromosomes is unmistakeably identifiable from its specific pattern of markers as a fusion of two ape chromosomes.
It should be noted that chromosomal duplications in plants don't seem to bother them much. Modern analyses show that plants have a startling disposition towards spontaneous duplication of their complete genomes, with evolution then winnowing down the duplications in the genome over time. For plants, over the long run at least, genome duplications are normal.
Mutations are happening all the time. Each one of us has about 175 mutations in the 7 billion bases in our genetic code. Every human is a mutant. The term "mutant" tends to suggest a monstrosity, a suggestion encouraged by the way monstrous mutations are played up in the news and in popular media, and the fact that modern medical research necessarily focuses on health problems caused by mutations -- cystic fibrosis and sickle-cell anemia, for example.
However, mutations are not necessarily harmful, and for that matter they're generally not very noticeable. This is because the mutations:
It should be noted that such "silent" mutations aren't necessarily completely silent, one reason being that the chemical reactivity of different triplets for the same base may differ enough to incorporate "delays" in the process of assembling the protein. The amino acid sequence remains the same, but with larger proteins the chain may fold up differently, affecting protein function. A very slight change in the genetic sequence could make a big difference in protein function.
In any case, not only are mutations generally nothing much to worry about, whether a mutation is "good" or "bad" can depend on circumstances -- as discussed later, sickle-cell anemia being a very good example.
It is true that the odds of obtaining any specific beneficial single-point mutation don't sound good. It is known from about a century of lab studies that a single-point mutation at any specific site in the genome will take place about once every 500 million births. Since there's four different bases, for a given locus there's three different possible single-point mutations from one base to one of three others, and restricting the mutation to one of those three bases triples the odds against it, up to one mutation per 1.5 billion offspring.
An examination of the details shows that even at those odds, it's not really a long shot. Humans and other mammals are "diploid" organisms, with duplicated genomes, which doubles the chance for getting the desired mutation, cutting the odds down to once per 750 million offspring. Of course, if the modified gene is dominant, it will be expressed in any organism that carries it; if it is recessive, it will only be expressed in organisms where both genes are modified -- the gene will be there, but it will only be seen one time out of four on the average.
Even once in 750 million births sounds outrageous, but microorganisms can achieve such populations in a small number of hosts in a short period of time. As far as macro-organisms go, it's a rare species that has less than a million members, and on the geological or evolutionary timescale, 750 generations amount to a blink of an eye. Of course, other parts of an organism's genome are mutating in parallel, and it's not like it will take 750 generations per mutation, one after another. The scramblings of sexual recombination permit mutations acquired in parallel by different members of the same species to gradually "gang up" under the unceasing supervision of natural selection.
* Although some sorts of mutations are more likely than others, and some sites or "hot spots" in a genome are more prone to mutations than others, mutations are effectively chance events, not occurring in a predictable fashion. However, in 1988, John Cairns (1922:2018), a prominent British molecular biologist, and his colleagues published a paper that seemed to challenge the chance nature of mutations, describing how bacteria placed under strong selection pressures adapted far faster than seemed likely by known processes of mutation. Cairns even wondered if this suggested some Lamarckian mechanism at work, that the mutations were somehow "directed".
There was lively debate over the matter, but it was eventually determined that the bacteria were not undergoing directed mutations -- instead, they were mutating at a vastly faster rate than normal, with the bacteria featuring a neat mechanism for accelerating the mutation rate when under stress, a scheme known as "hypermutability". A process called "gene amplification" was also discovered, in which a gene that is damaged is replicated at a greater rate than other genes.
In sum, instead of the bacteria winning at the evolutionary slot machine in violation of the odds, under pressure they were just playing the slot machine at a much faster rate -- and even without any change in the odds, the number of "wins" rose accordingly, the wastage of the losers not amounting to much since the attrition rate of the bacteria was higher than normal in the first place. The idea of directed mutation suffers from the clear failing of Lamarckism, suggesting that a bacterium with not much more ability to plan than a rock could, in response to a new and previously unknown threat, deliberately analyze the threat, determine what change in bacterial systems was required to meet it, and then tweak its genome accordingly.
Another curve thrown at genomics is epigenetics, mentioned earlier, the study of environmental influences on inheritance. Heredity is of course not just a matter of the genome, it also involves cellular machinery interpreting the genome -- and of course cells involved in reproduction can be altered by environmental processes, resulting in distinct differences in the next generation of an organism. We've been much about epigenetics in recent years, but claims that it "revolutionizes" our views of evolution are overblown. Nobody is particularly surprised by the concept that the environment in which cells exist can alter their expression of the genome. Epigenetics is likely less interesting in terms of its impact on evolutionary science than the microbiome.
* Despite the fact that the odds of obtaining a beneficial mutation are by no means unreasonable, creationists dismiss the idea, even concocting a doctrine known as "genetic entropy" to set up as a grand principle that "all mutations are harmful" and lead to the "inevitable" degradation of genomes.
Examination of the details suggests nothing of the sort. For example, in response to the famous story of how bacteria acquired the ability to digest nylon, some creationists claimed the bacteria picked up a plasmid through horizontal transfer from another bacteria that already had that capability. This is a bit unconvincing in the first place, since nylon is a synthetic substance that didn't exist a century ago; it's hard to imagine that some bacteria would be sitting around with a functional gene in "cold storage", just waiting for a material that didn't exist yet to come along. As discussed later, "genetic cold storage" is nonsense -- as such, it can't happen.
Still closer examination gave a neat picture of how the bacteria had acquired the ability to digest nylon through a mutation. The overall genetic structure of the nylon-digesting bacterium was the same as that of its non-nylon digesting ancestors, but it featured a "frame shift" mutation in a gene in one of its three plasmids. In a frame-shift mutation, a base is inserted (or deleted) from the gene; this throws off the sequence by which the DNA sequence is read by the protein-expression system, or in other words shifts its "reading frame" of base triplets and completely changes the expression of the gene.
Frame shifts tend to be catastrophic, but in the case of the nylon-digesting bacterium, it just luckily happened that the new protein expressed from the gene ended up being an enzyme, nylonase, that could digest nylon. Incidentally, there is more than one nylonase gene in the bacterium, but only one is needed to do the basic job -- the others seem to have been added later in gene duplications.
An unbelievable coincidence? In reality, nearly inevitable given the vast numbers of bacteria continuously replicating all around us -- and the fact that the nylonase that was produced did a pretty poor job, being only a fraction as efficient as the enzyme that had been originally produced. Give it time; evolutionary processes are designing bacteria with more effective nylonases on a step-by-step basis all the time.
BACK_TO_TOP* For a close-up on the impact of evolutionary genetic changes on a macroscale organism, consider the Antarctic icefish. It was formally described by a Norwegian biologist in the 1950s, who pointed out that it had a very strange feature: it didn't have blood as we understand it, instead literally having ice water running in its veins.
About 15 species of icefish are known. They are part of a larger group of cold-water fishes, the suborder "Notothenioidae", related to codfish and containing about 200 species. The icefish are very extreme variants of this suborder, being the only known vertebrates that do not have red blood. In all other vertebrate species, the blood is full of red blood cells, which are used to transport oxygen through the circulatory system. The red blood cells are loaded with hemoglobin molecules, which are composites of a set of similar proteins, the "globins", with a core molecule named "heme". It is the heme component which actually binds with oxygen, and which gives blood its red color.

Icefish do not have a trace of hemoglobin in their bodies. It wasn't until the 1990s that the story of the icefish was well understood. It was known before then that Antarctic waters had been cooling for over 50 million years, gradually driving fish species that couldn't stand the cold northward, leaving behind species that had obtained mechanisms to deal with the low temperatures.
At low temperatures, the viscosity of body fluids increases, making it harder to pump blood. One way of dealing with the viscosity problem is to cut the red blood cell count. Human blood is about 45% red blood cells by volume, while red-blooded Antarctic notothenioids have blood that's about 15% red blood cells by volume. The icefish have done this more than one better by not having any red blood cells at all -- in fact, their blood is only 1% cells by volume, the only cells being white blood cells used by the immune system.
So how can an icefish still be alive? In compensation for the loss of red blood cells, colder waters can absorb more oxygen -- in other words, all the icefish needs to pump is water to keep oxygen flowing to its brain and other organs. Cold water's still not as efficient as red blood cells in carrying oxygen, but the icefish keeps on going with larger gills; a bigger heart; a circulatory system with greater overall volume; and a network of capillaries under scaleless skin -- allowing it to soak up oxygen directly from the water around it.
The idiosyncrasies of the icefish are recorded in the patterns of their DNA. The genomes of their relatives have twin genes for production of globins, but in the icefish one of these genes is garbled into functionlessness by mutations and the other has simply disappeared. In addition, five of the icefish species also have a garbled and broken gene for production of "myoglobin", another heme-containing, oxygen-binding molecule found in muscles. Myoglobin binds oxygen more tightly than hemoglobin and is used to provide oxygen to muscles on demand; diving mammals like seals and dolphins have muscles that have very high proportions of myoglobin, allowing them to stay underwater for long periods of time and dying their muscles dark brown. The five icefish species with the broken myoglobin gene have pale muscles and a pale heart.
Production of myoglobin doesn't seem to impose any strong penalty on icefish in itself -- if it did, all icefish species would have probably lost it by now -- but with the adaptations of icefish to obtaining more oxygen, it doesn't provide any real advantage, either. When the myoglobin gene broke in a part of the icefish population, in effect they didn't notice it was gone, obtaining a slight advantage from not having to go to the effort of producing it to compensate for the disadvantages, if any, of losing it. Incidentally, this is known as "relaxed selection".
There are other unique and tweaky changes in the icefish. Cells make use of a class of structural proteins that form "microtubules" -- which in other vertebrate species tend to break down at low temperatures. All the notothenioids have microtubules that feature minor changes that ensure their low-temperature stability, the changes reflected in the genes that encode the microtubules.
The notothenioids also have a particularly interesting and unique adaptation to low temperatures: their blood is full of "antifreeze" proteins. These "antifreeze" proteins consist only of 4 to 55 "repeats" of a chain of just three amino acids. Analysis shows that the genes for the antifreeze proteins seem to have evolved from a digestive enzyme, with a chunk of the gene for the enzyme breaking off and being relocated on the fish genome. The clue is that the antifreeze gene includes a distinctive chunk of noncoding DNA that is also associated with the digestive enzyme.
Evolution envisions a branching tree of species flowing out from common ancestry, and the pattern of distribution of these various cold-water adaptations gives a strong clue to their history of emergence. The notothenioid species, with their antifreeze proteins and modified microtubules, are estimated from DNA rates of change to have emerged about 25 million years ago. Red cell counts gradually declined, and then the icefish lost the ability to make hemoglobin, which occurred maybe 8 million years ago. Since only a third of the icefish have lost the myoglobin gene, that change occurred more recently still, and in fact can be regarded as a trait that is still evolving.
Under natural selection, the icefish didn't get rid of their hemoglobin genes: the genes broke on their own and it just so happened that the icefish got along fine, even better, without them. Even hardcore advocates of MET have a tendency to say things such as the icefish "invented" the antifreeze proteins, or they "deleted" the genes for hemoglobin production. The fish didn't invent or delete anything: they don't have the brains to add two and two to get four, they could hardly have redesigned their own genome.
They instead acquired new features that proved useful, and lost those that weren't useful any more. Mutations occurred by sheer chance that turned out to be beneficial to those fish that possessed them, with the fish winning a throw of the dice in which they had no say or even awareness. Of course, there was a downside to the ticket they obtained in the evolutionary lottery: icefish are superbly adapted to life in cold waters to the extent that they will die in warm waters, because they will not be able to pump adequate oxygen through their circulatory system.
BACK_TO_TOP* As noted earlier, evolution works in general by small gradual changes, gradually accumulating major changes in form by a process of "compounded interest". Darwin's focus on such gradual change wasn't entirely accepted even by his allies, who still held on to Lamarckian and saltationist ideas. Mendel's work on heredity would have helped, but it remained unknown for decades. As discussed previously, even after it was rediscovered after the turn of the century, notions of saltationism persisted for a time.
A Harvard biologist named William E. Castle (1867:1962) decided to test the concept of gradual change through selective breeding of a species of rat known as the "hooded rat". The hooded rat has a pattern of light fur on its body and dark fur over its head; Castle and his students found, somewhat to their surprise, that they could change the dark coloration pattern by slow increments to produce a dark stripe down the back, continuing until they had rats with no light fur at all. Maybe the idea of slow, incremental changes wasn't so unrealistic after all. Darwin, with his knowledge of pigeon breeding, might well have thought, though he would certainly have been too polite to say: "I told you so."
Another one of the skeptics of the period, a pioneering geneticist named Reginald C. Punnett (1875:1967), was interested in mimicry in butterflies -- the tendency of some butterflies that taste good to imitate the color patterns of butterflies that taste bad, discouraging predators. In the course of his studies, Punnett wondered how long it would take natural selection to propagate an advantageous trait through a population and how long it would take it to eliminate a disadvantageous trait. He worked with a mathematician named H.T.J. Norton to do the calculations -- and the results showed that the number of generations was actually surprisingly small.
In modern terms, the basis of such calculations are what are called "selection coefficients". Suppose a "normal" member of a population of one species can produce 100 offspring. If a mutant variant can produce 101 offspring with a level of health equal to that of the normal member, then the mutant has a selection coefficient of +0.01. If a mutant variant can produce only 99 offspring with a level of health equal to that of the normal variant, then the mutant has a selection coefficient of -0.01.
Norton's calculations showed that if eight members of a population of 1,000 with a mutant trait had a selection coefficient of +0.01 relative to the rest of the population, then the number of individuals in that population with that trait would be more than 90% in 3,000 generations. If the selection coefficient was +0.1, then it would only take 300 generations to take over.
Traits themselves tend to compound as well. Suppose individuals in a population get a selective advantage from being bigger. A change of 0.2% -- two millimeters for a one-meter-long plant or animal -- would be imperceptible from one generation to the next, but with compounding it would increase size by 50% in 200 generations.
* This is all very interesting, but mathematical analysis simply shows what can happen given basic assumptions, not actually what does happen, which has to be observed in the real world. The difficulty is that it's very hard to pick out slight differences from one generation to the next, since individuals vary from one another. There are well-defined statistical methods to determine the degree of confidence in samplings -- the details aren't particularly interesting here, enough to say that the larger the sample and the bigger the change being measured, the higher the confidence.
That means the best-documented cases of natural selection being observed in the wild occur when selection is strong and rapid. The classic example is the British peppered moth. J.B.S. Haldane estimated that the selection coefficient of the moth was about -0.2. That's a pretty big selection coefficient, as it would be expected given the rapid adjustment in the moth population.
The peppered moth is one of the most famous example of evolutionary change, but field biologists have detailed other examples. Modern studies of the shift of populations of the Galapagos finches during drought years demonstrated that a clear selective advantage could be obtained by a change of beak length of only half a millimeter a year.
Another example is the three-spine stickleback fish. During the ice ages, ocean-living sticklebacks invaded fresh water lakes and streams, only to become reproductively isolated after the end of the ice ages. While oceanic sticklebacks have a protective row of more than 30 bony plates running up their sides, the freshwater sticklebacks apparently don't need them -- and so have no more than nine, sometimes no, armor plates on each side. After a chemical eradication program killed off the stickleback population of Loberg Lake in Alaska, in 1982 the lake was recolonized by oceanic sticklebacks. From 1990 to 2001, regular sampling showed that the frequency of the oceanic form of the stickleback went from 100% to 11%, with relatively unarmored sticklebacks making up the rest of the population.
Why did the lake sticklebacks "decide" to start getting rid of their armor plates? They didn't decide anything -- a random mutation simply got rid of them, and the fish with the mutation outbred those who didn't have the mutation. It's the same process as created the icefish. A random mutation that would have been harmful to a normal fish -- causing the loss of the ability to make hemoglobin -- was a positive benefit to the icefish. That particular mutation was only part of a cumulative series of mutations that gave the icefish a bigger heart, bigger gills, a heftier circulatory system, with each change arising one at a time, being screened out by the "trial & error" process of natural selection.
* For an example of the potential usefulness of mutations, consider a population of light-colored mice living in a sandy environment. If a mutation produces a dark-colored mouse, it will be at a severe disadvantage against predators and will likely die out quickly. Suppose lava flows then run across parts of the region. Light-colored mice will be at a disadvantage, while the dark-colored mice will have an advantage.
It won't take that long to get dark-colored mice, either. Fur darkness is due to a substance called "melanin", produced by cells called "melanocytes". The mouse pituitary gland creates a melanin-stimulating hormone that binds to a "melanocortin-1 receptor (MC1R)" protein on the melanocytes to ramp up melanin production. The MC1R receptor is produced from an MC1R gene. About ten different mutations can change MC1R from specifying light fur color to dark fur color, and a mouse has two copies of the gene. Given that a specific single-point mutation occurs in about one in 1.5 billion births, then about 1 in 75 million mice with light fur color will obtain a gene to produce offspring with dark fur color.
Consider even a small population of 10,000 mice, with each female (half the population) bearing a litter of five mice a year. This means 25,000 babies a year, with a new generation every year, and that a black mouse will arise, on the average, once in three thousand years. Kick up the population to 100,000, and the dark mutant will arise once every three centuries. This is much better odds than playing the lottery.
Now let's consider how long it takes for the dark color gene to propagate though the population. This matter is well-understood by population geneticists. The time in generations T is a function of the selective advantage of the black fur trait, S, and the size of the effective breeding population Ne:
T = (2 / S) * NLOG(2 * Ne)
Given an Ne of 10,000 individuals, then the number of generations corresponding to given selective advantages is:
S = 0.001: 19,807 generations S = 0.01: 1,981 generations S = 0.05: 396 generations S = 0.1: 198 generations S = 0.2: 99 generations
The calculation may be complicated by other factors, such as the odds that mice with the black mutation may not pass it down -- either the mutant gets killed off or the mutant gene gets lost in the shuffle of chromosomes. However, if the gene dies out in one pass, it will certainly come back again later, and the odds of it losing out a second time are very low.
This example of light-colored and dark-colored mice is based in the real world. In Arizona, populations of light-colored and dark-colored mice both live in the desert, with the light-colored mice in sandy areas and dark-colored mice in areas featuring lava flows. The precise mutation that causes these two variants to differ is known.
It should be noted that even if negative mutations are far more common than positive mutations, that makes no problems for MET. Under MET, populations of species are always undergoing attrition, and even if 99 out of 100 mutations cause problems, those members of those species are afflicted simply become part of the normal attrition. That 1 in 100 mutation, in contrast, has a good chance of being carried on and propagating. A scarcity of positive mutations does not stop evolution; it just slows it down, and nobody claims it should work overnight to begin with.
BACK_TO_TOP* One of the more intriguing revelations of the genetic analysis of organisms is that a pool of functionally identical genes are shared among them. Given the known rates of mutations, it would seem that in the vast millions of years life has been around on Earth, random mutations would have ensured that there would be no genetic similarity between distantly related species of organisms. In reality, there are many similarities in the genes of distantly related species. In fact, there are about 500 genes that are universal to all forms of life: since the likelihood of two different organisms deriving genes with highly similar codes by sheer chance is vanishingly small, by implication these genes have been around since nearly the beginning.
However, since genes are always mutating, how could these "immortal genes" have been preserved? The answer's simple: the genes are so important that any major change in them is at the very least disadvantageous, and at worst fatal. In evolutionary terms, these genes correspond to very steep "fitness functions"; organisms can't be pushed away from those functions and survive over the long run, if they can live at all. In the genome, the immortal genes are the ultimate "star players", spreading their presence over a wide number of species and maintaining their star status indefinitely.
It should also be noted that immortal genes are only functionally identical: they produce proteins that work the same way, but these proteins may have slightly different amino acids in some places, though they will share a common subset. That corresponds to variations in the gene's coding, and even when two genes give exactly the same amino acid sequence, they may feature silent mutations and not have the same base sequences. Even more significantly, there is a branching tree of variations among the immortal genes of different species that tracks their taxonomic relationships: for example, the common immortal genes of plants are more similar to each other than they are of those of a human being.
Richard Dawkins once pointed out that immortal genes provide an extremely powerful argument in favor of evolutionary common descent. Going back to the analogy of the evolution of languages, two highly dissimilar languages such as Japanese and English have almost nothing in common, except for modern borrowings between the languages. In contrast, as Dawkins pointed out, there are entire "paragraphs" in the genetic codes of bacteria and humans that are functionally the same.
BACK_TO_TOP